The inexorable increase of biologic exposure in paediatric inflammatory bowel disease: a Scottish, population-based, longitudinal study
- PMID: 36196524
- PMCID: PMC9828169
- DOI: 10.1111/apt.17217
The inexorable increase of biologic exposure in paediatric inflammatory bowel disease: a Scottish, population-based, longitudinal study
Abstract
Background: The use of biologics in paediatric-onset inflammatory bowel disease (PIBD) is rapidly changing.
Aims: To identify the incidence and prevalence of biologic use within Scottish PIBD services, and to describe patient demographics and outcomes for those patients who required escalation of therapy beyond anti-tumour necrosis factor alpha (anti-TNFα) agents METHODS: We captured a nationwide cohort of prospectively identified patients less than 18 years of age with PIBD (A1 phenotype; diagnosed <17 years of age) within paediatric services over a 4.5-year period (1 January 2015-30 June 2019). All patients who received infliximab, adalimumab, vedolizumab or ustekinumab during the study period and/or received their first dose of these biologics were audited retrospectively.
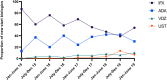
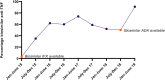
Results: Scotland-wide PIBD-prevalence cases increased from 554 to 644 over the study period. A total of 495 incident new-start biological therapies were commenced on 403 PIBD patients: 295 infliximab (60%), 161 adalimumab (32%), 24 vedolizumab (5%) and 15 ustekunumab (3%). The proportion of new-start biologics changed with infliximab initiation rates decreasing (87%-54%) while adalimumab (13%-31%), vedolizumab (0%-9%) and ustekinumab (0%-6%) all increased. The incidence rate (first dose of new biologic not including biosimilar switch) increased from 6.9% to 8.1% over the study period and point prevalence rates (any biologic use) increased from 20.2% to 43.5% - an average annual percentage increase of 20%. Biosimilar penetration of new-start anti-TNFα agents increased from 3% to 91%. Demographics and outcomes of those patients receiving vedolizumab and ustekinumab were similar.
Conclusions: Complete accrual of Scottish nationwide biologic usage within paediatric services demonstrates a rapidly changing, inexorably increasing PIBD biologics landscape.
© 2022 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures
Comment in
-
Editorial: implications and future perspectives of the increase in biologics use in paediatric inflammatory bowel disease.Aliment Pharmacol Ther. 2022 Dec;56(11-12):1619-1620. doi: 10.1111/apt.17243. Aliment Pharmacol Ther. 2022. PMID: 36352749 No abstract available.
References
-
- Ghione S, Sarter H, Fumery M, Armengol‐Debeir L, Savoye G, Ley D, et al. Dramatic increase in incidence of ulcerative colitis and Crohn's disease (1988‐2011): a population‐based study of French adolescents. Am J Gastroenterol. 2018;113:265–72. - PubMed
-
- Burgess C. Paediatric patients (less than age of 17 years) account for less than 1.5% of all prevalent inflammatory bowel disease cases. J Pediatr Gastroenterol Nutr. 2020; 71(4): 521–23. - PubMed
-
- van Limbergen J., Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, et al. Definition of phenotypic characteristics of childhood‐onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22. - PubMed
-
- Kuenzig ME, Fung SG, Marderfeld L, JWY Mak, Kaplan GG, Ng SC, et al. Twenty‐first century trends in the global epidemiology of pediatric‐onset inflammatory bowel disease: systematic review. Gastroenterology 2022;162: 1147–1159. - PubMed
-
- Henderson P, Hansen R, Cameron FL, Gerasimidis K, Rogers P, Bisset MW, et al. Rising incidence of pediatric inflammatory bowel disease in Scotland. Inflamm Bowel Dis. 2012;18:999–1005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources