Treatment adaptations and outcomes of patients experiencing inflammatory bowel disease flares during the early COVID-19 pandemic: the PREPARE-IBD multicentre cohort study
- PMID: 36196569
- PMCID: PMC9874879
- DOI: 10.1111/apt.17223
Treatment adaptations and outcomes of patients experiencing inflammatory bowel disease flares during the early COVID-19 pandemic: the PREPARE-IBD multicentre cohort study
Abstract
Background: The COVID-19 pandemic offered a unique opportunity to understand inflammatory bowel disease (IBD) management during unexpected disruption. This could help to guide practice overall.
Aims: To compare prescribing behaviour for IBD flares and outcomes during the early pandemic with pre-pandemic findings METHODS: We performed an observational cohort study comprising patients who contacted IBD teams for symptomatic flares between March and June 2020 in 60 National Health Service trusts in the United Kingdom. Data were compared with a pre-pandemic cohort after propensity-matching for age and physician global assessment of disease activity.
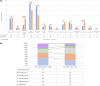
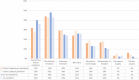
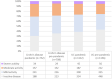
Results: We included 1864 patients in each of the pandemic and pre-pandemic cohorts. The principal findings were reduced systemic corticosteroid prescription during the pandemic in Crohn's disease (prednisolone: pandemic 26.5% vs. 37.1%; p < 0.001) and ulcerative colitis (UC) (prednisolone: pandemic 33.5% vs. 40.7%, p < 0.001), with increases in poorly bioavailable oral corticosteroids in Crohn's (pandemic 15.6% vs. 6.8%; p < 0.001) and UC (pandemic 11.8% vs. 5.2%; p < 0.001). Ustekinumab (Crohn's and UC) and vedolizumab (UC) treatment also significantly increased. Three-month steroid-free remission in each period was similar in Crohn's (pandemic 28.4% vs. 32.1%; p = 0.17) and UC (pandemic 36.4% vs. 40.2%; p = 0.095). Patients experiencing a flare and suspected COVID-19 were more likely to have moderately-to-severely active disease at 3 months than those with a flare alone.
Conclusions: Despite treatment adaptations during the pandemic, steroid-free outcomes were comparable with pre-pandemic levels, although concurrent flare and suspected COVID-19 caused worse outcomes. These findings have implications for IBD management during future pandemics and for standard practice.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
SS holds research grants from Biogen, Takeda, AbbVie, Tillotts Pharma, Ferring and Biohit; served on the advisory boards of Takeda, AbbVie, Merck, Ferring, Pharmacocosmos, Warner Chilcott, Janssen, Falk Pharma, Biohit, TriGenix, Celgene and Tillots Pharma; and has received speaker fees from AbbVie, Biogen, AbbVie, Janssen, Merck, Warner Chilcott and Falk Pharma. GJW has served as a speaker and/or advisory board member for AbbVie, Falk and Janssen. He has had support to attend meetings from AbbVie, Falk, Janssen and Norgine. His department has received research funding from Tillotts. NAK has served as a speaker and/or advisory board member for Allergan, Falk, Janssen, Mylan, Pharmacosmos, Takeda and Tillotts. He has had support to attend meetings from AbbVie, Falk, Janssen and Norgine. His department has received research funding from AbbVie, Celgene, Celtrion, MSD, Napp, Pfizer, Pharmacosmos and Takeda. SrS has received speaker fees from MSD, Actavis, Abbvie, Dr Falk pharmaceuticals, Shire and received educational grants from MSD, Abbvie, Actavis and is an advisory board member for Celltrion, Dr Falk pharmaceuticals and Vifor pharmaceuticals. CAL has received research support and/or has received fees for delivery of non‐promotional education from: Genentech, Janssen, Takeda, Abbvie, Dr Falk, AstraZeneca, Eli Lilly, Orion, Pfizer, Roche, Sanofi Aventis, Ferring, UCB and Biogen. MJB has received research grants from Vifor International, Pharmacosmos and Tillots Pharma; has received speaker fee from Abbvie and Vifor International; has been an advisory board member for Tillots Pharma, Vifor International; and received travel/conference expenses from Vifor International, Abbvie and Tillots Pharma. AJK has served on the advisory boards for Abbvie, Janssen and BMS Celgene and has received speaker fees from Takeda, Pfizer and Janssen; and received travel/conference expenses from Tillotts, Janssen, Abbvie and Shield Therapeutics. KP has received honoraria for educational meetings and speaker fees from Abbvie, Janssen, Takeda, DrFalk and Ferring. KP has received Advisory Board fees from Abbvie and Janssen. TEC has received speaker fees from Celltrion. LCH, HAG, SJM, AS have no conflicts of interest to declare.
Figures


Comment in
-
Editorial: the impact of COVID on the management of IBD flares: different care but similar outcomes-authors' reply.Aliment Pharmacol Ther. 2023 Jan;57(1):156-157. doi: 10.1111/apt.17293. Epub 2022 Dec 5. Aliment Pharmacol Ther. 2023. PMID: 36468219 Free PMC article.
-
Editorial: the impact of COVID on management of IBD flares-different care but similar outcomes.Aliment Pharmacol Ther. 2023 Jan;57(1):154-155. doi: 10.1111/apt.17259. Aliment Pharmacol Ther. 2023. PMID: 36480717 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
