Booster Vaccination Against SARS-CoV-2 Induces Potent Immune Responses in People With Human Immunodeficiency Virus
- PMID: 36196614
- PMCID: PMC9619587
- DOI: 10.1093/cid/ciac796
Booster Vaccination Against SARS-CoV-2 Induces Potent Immune Responses in People With Human Immunodeficiency Virus
Abstract
Background: People with human immunodeficiency virus (HIV) on antiretroviral therapy (ART) with good CD4 T-cell counts make effective immune responses following vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). There are few data on longer term responses and the impact of a booster dose.
Methods: Adults with HIV were enrolled into a single arm open label study. Two doses of ChAdOx1 nCoV-19 were followed 12 months later by a third heterologous vaccine dose. Participants had undetectable viraemia on ART and CD4 counts >350 cells/µL. Immune responses to the ancestral strain and variants of concern were measured by anti-spike immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA), MesoScale Discovery (MSD) anti-spike platform, ACE-2 inhibition, activation induced marker (AIM) assay, and T-cell proliferation.
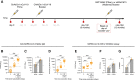
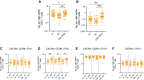
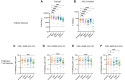
Findings: In total, 54 participants received 2 doses of ChAdOx1 nCoV-19. 43 received a third dose (42 with BNT162b2; 1 with mRNA-1273) 1 year after the first dose. After the third dose, total anti-SARS-CoV-2 spike IgG titers (MSD), ACE-2 inhibition, and IgG ELISA results were significantly higher compared to Day 182 titers (P < .0001 for all 3). SARS-CoV-2 specific CD4+ T-cell responses measured by AIM against SARS-CoV-2 S1 and S2 peptide pools were significantly increased after a third vaccine compared to 6 months after a first dose, with significant increases in proliferative CD4+ and CD8+ T-cell responses to SARS-CoV-2 S1 and S2 after boosting. Responses to Alpha, Beta, Gamma, and Delta variants were boosted, although to a lesser extent for Omicron.
Conclusions: In PWH receiving a third vaccine dose, there were significant increases in B- and T-cell immunity, including to known variants of concern (VOCs).
Keywords: SARS-CoV-2; T-cell responses; antibody responses; people with HIV; vaccination.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

