Fenfluramine provides clinically meaningful reduction in frequency of drop seizures in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study
- PMID: 36196777
- PMCID: PMC10099582
- DOI: 10.1111/epi.17431
Fenfluramine provides clinically meaningful reduction in frequency of drop seizures in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study
Erratum in
-
Correction to "Fenfluramine provides clinically meaningful reduction in frequency of drop seizures in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study".Epilepsia. 2024 Jul;65(7):2179. doi: 10.1111/epi.17999. Epub 2024 May 30. Epilepsia. 2024. PMID: 38813894 Free PMC article. No abstract available.
Abstract
Objective: This study was undertaken to evaluate the long-term safety and effectiveness of fenfluramine in patients with Lennox-Gastaut syndrome (LGS).
Methods: Eligible patients with LGS who completed a 14-week phase 3 randomized clinical trial enrolled in an open-label extension (OLE; NCT03355209). All patients were initially started on .2 mg/kg/day fenfluramine and after 1 month were titrated by effectiveness and tolerability, which were assessed at 3-month intervals. The protocol-specified treatment duration was 12 months, but COVID-19-related delays resulted in 142 patients completing their final visit after 12 months.
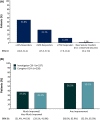
Results: As of October 19, 2020, 247 patients were enrolled in the OLE. Mean age was 14.3 ± 7.6 years (79 [32%] adults) and median fenfluramine treatment duration was 364 days; 88.3% of patients received 2-4 concomitant antiseizure medications. Median percentage change in monthly drop seizure frequency was -28.6% over the entire OLE (n = 241) and -50.5% at Month 15 (n = 142, p < .0001); 75 of 241 patients (31.1%) experienced ≥50% reduction in drop seizure frequency. Median percentage change in nondrop seizure frequency was -45.9% (n = 192, p = .0038). Generalized tonic-clonic seizures (GTCS) and tonic seizures were most responsive to treatment, with median reductions over the entire OLE of 48.8% (p < .0001, n = 106) and 35.8% (p < .0001, n = 186), respectively. A total of 37.6% (95% confidence interval [CI] = 31.4%-44.1%, n = 237) of investigators and 35.2% of caregivers (95% CI = 29.1%-41.8%, n = 230) rated patients as Much Improved/Very Much Improved on the Clinical Global Impression of Improvement scale. The most frequent treatment-emergent adverse events were decreased appetite (16.2%) and fatigue (13.4%). No cases of valvular heart disease (VHD) or pulmonary arterial hypertension (PAH) were observed.
Significance: Patients with LGS experienced sustained reductions in drop seizure frequency on fenfluramine treatment, with a particularly robust reduction in frequency of GTCS, the key risk factor for sudden unexpected death in epilepsy. Fenfluramine was generally well tolerated; VHD or PAH was not observed long-term. Fenfluramine may provide an important long-term treatment option for LGS.
Keywords: Lennox-Gastaut syndrome; developmental and epileptic encephalopathies; fenfluramine; long-term open-label extension.
© 2022 UCB and The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
K.G.K. discloses research grants from Zogenix and Pediatric Epilepsy Research Foundation during the conduct of the study, as well as from the Colorado Department of Public Health and West Therapeutics, and received other support as a data safety monitoring board member and from Greenwich Pharmaceuticals outside the submitted work. I.E.S. discloses compensation from Zogenix during the conduct of the study, has received other support from Zynerba Pharmaceuticals, GW Pharmaceuticals, Ovid Therapeutics, Marinus, and Ultragenyx, has received personal fees and other compensation from UCB, has received personal fees from GlaxoSmithKline, Eisai, BioMarin, Nutricia, and Xenon Pharmaceuticals outside the submitted work, and has received research funding from the National Health and Medical Research Council, Health Research Council of New Zealand, and National Institutes of Health. B.C. discloses research funding from Brabant and Zogenix, has served as consultant for Brabant and Zogenix, is a patent‐holder for ZX008, and potentially benefits financially from a royalty arrangement that is related to this research if Zogenix is successful in marketing its product, fenfluramine, with the terms of this arrangement reviewed and approved by the cobeneficiary, KU Leuven University/Antwerp University Hospital. J.S. discloses research grants from Stoke, Marinus, Zogenix, and BioPharm, served as consultant/advisor for the Dravet Syndrome Foundation, Epygenix, Encoded, GW Pharmaceuticals, Asceneuron, Longboard Pharmaceuticals, Knopp Biosciences, and Neurocrine, owns stock options in Epygenix, has received travel support from Zogenix, and has served as a reviewer for the Epilepsy Study Consortium. L.L. discloses grants, personal fees, and other support from, and has served as consultant/speaker for, Zogenix during the conduct of the study; has received other support from and served as consultant/speaker for LivaNova; has received grants and other support from, and served as consultant/speaker for, UCB; has received other support from and served as consultant/speaker for Shire; has served as consultant/speaker for Eisai, Brabant, and Ovid, outside the submitted work; holds a patent for ZX008 for the treatment of Dravet syndrome and infantile epilepsies assigned to his institution and licensed to Zogenix; and potentially benefits financially from a royalty arrangement that is related to this research if Zogenix is successful in marketing its product, fenfluramine, with the terms of this arrangement reviewed and approved by the cobeneficiary, KU Leuven University/Antwerp University Hospital. R.G. discloses research grants from Zogenix during the conduct of the study, has served as speaker/consultant for Zogenix outside the submitted work, has served as an investigator for studies with Biocodex, UCB, Angelini, and Eisai, and has served as speaker and advisory board member for Biocodex, Novartis, BioMarin, and GW Pharma outside the submitted work. S.M.Z. discloses research support from Epilepsy Research UK, Dravet Syndrome UK, and Zogenix, and has served as consultant/speaker and advisory board member for GW Pharma, Encoded Therapeutics, Stoke Therapeutics, Eisai, UCB, Jaguar Gene Therapy, Arvelle, and Zogenix. R.N. discloses research funding from Eisai, GW Pharma, Novartis, Shire, and Zogenix, has served as consultant/advisor for Eisai, Biogen, GW Pharma, Novartis, Shire, and Zogenix, and has served as speaker for Advicenne, Eisai, BioMarin, GW Pharma, Novartis, and Zogenix. K.R. has received honoraria for educational symposia, advisory boards, and/or consultancy work from Eisai, LivaNova, Medlink Neurology, Novartis, and UCB Australia. Her institution has supported clinical trials for Biogen Idec Research, DSLP, Eisai, Epigenyx Therapeutics, GW Research, Janssen‐Cilag, Marinus Pharmaceuticals, Medicure International, LivaNova, Neurocrine Biosciences, Noema Pharma, Novartis, SK Lifesciences, UCB Australia, UCB Biopharma SRL, and Zogenix. A.A. discloses personal fees from, owns stock in, and was an employee of Zogenix, with patents pending. M.L. discloses personal fees from, owns stock in, and was an employee of Zogenix, with patents pending during the time the research was conducted (at time of publication, reported independent consultancy for Zogenix). G.M.F., B.S.G., A.R.G., and S.P. disclose personal fees from, own stock in, and/or are/were employees of Zogenix (now a part of UCB), with patents pending. R.D. has served as speaker for LivaNova, Eisai, and Lundbeck, and has served as an investigator for LivaNova, Eisai, Global Pharmaceuticals, Lundbeck, Pfizer, UCB, and Zogenix. A.G.‐N. discloses personal fees or research grants from Arvelle Therapeutics, Bial, Biocodex, Eisai, Esteve, GW Pharma, GW Research, PTC Therapeutics, Sanofi, Stoke, UCB, and Zogenix. K.C.N. has no conflict of interest to disclose. D.D. has served as consultant for Zogenix.
Figures

References
-
- Gastaut H, Roger J, Soulayrol R, Tassinari CA, Regis H, Dravet C, et al. Childhood epileptic encephalopathy with diffuse slow spike‐waves (otherwise known as “petit mal variant”) or Lennox syndrome. Epilepsia. 1966;7(2):139–79. - PubMed
-
- Arzimanoglou A, French J, Blume WT, Cross JH, Ernst JP, Feucht M, et al. Lennox‐Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009;8(1):82–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

