Coordinated metabolic transitions and gene expression by NAD+ during adipogenesis
- PMID: 36197339
- PMCID: PMC9538974
- DOI: 10.1083/jcb.202111137
Coordinated metabolic transitions and gene expression by NAD+ during adipogenesis
Abstract
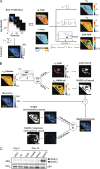
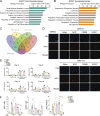
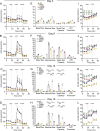
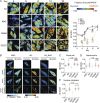
Adipocytes are the main cell type in adipose tissue, which is a critical regulator of metabolism, highly specialized in storing energy as fat. Adipocytes differentiate from multipotent mesenchymal stromal cells (hMSCs) through adipogenesis, a tightly controlled differentiation process involving close interplay between metabolic transitions and sequential programs of gene expression. However, the specific gears driving this interplay remain largely obscure. Additionally, the metabolite nicotinamide adenine dinucleotide (NAD+) is becoming increasingly recognized as a regulator of lipid metabolism, and a promising therapeutic target for dyslipidemia and obesity. Here, we explored how NAD+ bioavailability controls adipogenic differentiation from hMSC. We found a previously unappreciated repressive role for NAD+ on adipocyte commitment, while a functional NAD+-dependent deacetylase SIRT1 appeared crucial for terminal differentiation of pre-adipocytes. Repressing NAD+ biosynthesis during adipogenesis promoted the adipogenic transcriptional program, while two-photon microscopy and extracellular flux analyses suggest that SIRT1 activity mostly relies on the metabolic switch. Interestingly, SIRT1 controls subcellular compartmentalization of redox metabolism during adipogenesis.
© 2022 Sánchez-Ramírez et al.
Figures







Comment in
-
Temporal and spatial metabolite dynamics impart control in adipogenesis.J Cell Biol. 2022 Dec 5;221(12):e202210021. doi: 10.1083/jcb.202210021. Epub 2022 Nov 21. J Cell Biol. 2022. PMID: 36409212 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

