Falling treatment uptake in the hepatitis C care cascade is a growing threat to achieving elimination
- PMID: 36197840
- PMCID: PMC10091771
- DOI: 10.1111/jvh.13757
Falling treatment uptake in the hepatitis C care cascade is a growing threat to achieving elimination
Abstract
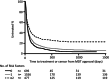
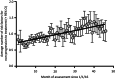
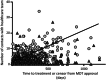
Most high-income countries are not on track to achieve the World Health Organization hepatitis C elimination targets. As elimination programmes assess growing proportions of patients in community-based pathways, rates of treatment uptake may fall. We aimed to identify factors associated with DAA treatment uptake and measure changes in their prevalence over time. We performed a time-to-treatment analysis on 2728 patients approved for hepatitis C Direct-Acting Antiviral treatment in the North Central London region between January 2016 and October 2019. We investigated the association between treatment uptake and factors including assessment/treatment setting (hospital, drug service or prison), patient age, gender, injection drug use, harmful alcohol use, cirrhosis status and previous treatment. The likelihood of treatment uptake was reduced by three independent risk factors. These included assessment setting: prison-based or drug-service pathways (aHR 0.29 or 0.81 vs. hospital outpatient pathway, 95% CI 0.21-0.40 and 0.70-0.94 respectively, p < .001); being UK-born (aHR 0.89 vs. non-UK born, 0.82-0.98, p = .01); and history of harmful alcohol use (aHR 0.84 vs. no history, 0.72-0.99, p = .04). The average number of these risk factors for not starting treatment per patient increased over time (R2 = 0.66 p < .001). Independent of these, there was an additional 5% reduction in rate of treatment initiation in each successive year of the programme (aHR 0.95, 0.91-0.99, p = .02). In conclusion, disengagement from care before treatment uptake was found to be a growing threat to elimination. Despite provision of community-based test-to-cure pathways, there are persistent barriers to treatment uptake and these are increasing over time.
Keywords: antiviral agents; hepatitis C; intravenous; risk factors; substance abuse; time-to-treatment.
© 2022 The Authors. Journal of Viral Hepatitis published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr Smith reports grants from ViiV Healthcare and personal fees from Gilead Sciences Ltd, outside the submitted work. The other authors declare no competing interests.
Figures
References
-
- World Health Organization . Global Health sector strategy on viral hepatitis 2016‐21: Towards Ending Viral Hepatitis. World Health Organization; 2016.
-
- Ward Z, Platt L, Sweeney S, et al. Impact of current and scaled‐up levels of hepatitis C prevention and treatment interventions for people WHO inject drugs in three UK settings—what is required to achieve the WHO's HCV elimination targets? Addiction. 2018;113(9):1727‐1738. doi: 10.1111/add.14217 - DOI - PMC - PubMed
-
- Harris HE, Costella A, Croxford S, et al. Hepatitis C in the UK, 2020: Working to Eliminate Hepatitis C as a Major Public Health Threat; 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

