miR-275/305 cluster is essential for maintaining energy metabolic homeostasis by the insulin signaling pathway in Bactrocera dorsalis
- PMID: 36197879
- PMCID: PMC9534453
- DOI: 10.1371/journal.pgen.1010418
miR-275/305 cluster is essential for maintaining energy metabolic homeostasis by the insulin signaling pathway in Bactrocera dorsalis
Abstract
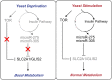
Increasing evidence indicates that miRNAs play crucial regulatory roles in various physiological processes of insects, including systemic metabolism. However, the molecular mechanisms of how specific miRNAs regulate energy metabolic homeostasis remain largely unknown. In the present study, we found that an evolutionarily conserved miR-275/305 cluster was essential for maintaining energy metabolic homeostasis in response to dietary yeast stimulation in Bactrocera dorsalis. Depletion of miR-275 and miR-305 by the CRISPR/Cas9 system significantly reduced triglyceride and glycogen contents, elevated total sugar levels, and impaired flight capacity. Combined in vivo and in vitro experiments, we demonstrated that miR-275 and miR-305 can bind to the 3'UTR regions of SLC2A1 and GLIS2 to repress their expression, respectively. RNAi-mediated knockdown of these two genes partially rescued metabolic phenotypes caused by inhibiting miR-275 and miR-305. Furthermore, we further illustrated that the miR-275/305 cluster acting as a regulator of the metabolic axis was controlled by the insulin signaling pathway. In conclusion, our work combined genetic and physiological approaches to clarify the molecular mechanism of metabolic homeostasis in response to different dietary stimulations and provided a reference for deciphering the potential targets of physiologically important miRNAs in a non-model organism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Identification and profiling of Bactrocera dorsalis microRNAs and their potential roles in regulating the developmental transitions of egg hatching, molting, pupation and adult eclosion.Insect Biochem Mol Biol. 2020 Dec;127:103475. doi: 10.1016/j.ibmb.2020.103475. Epub 2020 Oct 13. Insect Biochem Mol Biol. 2020. PMID: 33059019
-
miR-8-3p regulates mitoferrin in the testes of Bactrocera dorsalis to ensure normal spermatogenesis.Sci Rep. 2016 Mar 2;6:22565. doi: 10.1038/srep22565. Sci Rep. 2016. PMID: 26932747 Free PMC article.
-
Adipokinetic hormone receptor gene identification and its role in triacylglycerol mobilization and sexual behavior in the oriental fruit fly (Bactrocera dorsalis).Insect Biochem Mol Biol. 2017 Nov;90:1-13. doi: 10.1016/j.ibmb.2017.09.006. Epub 2017 Sep 15. Insect Biochem Mol Biol. 2017. PMID: 28919559
-
MicroRNAs in metabolic disease.Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):178-85. doi: 10.1161/ATVBAHA.112.300144. Arterioscler Thromb Vasc Biol. 2013. PMID: 23325474 Free PMC article. Review.
-
Interplay between microRNAs and the STAT3 signaling pathway in human cancers.Physiol Genomics. 2013 Dec 15;45(24):1206-14. doi: 10.1152/physiolgenomics.00122.2013. Epub 2013 Nov 5. Physiol Genomics. 2013. PMID: 24192393 Review.
Cited by
-
Developmental Expression Patterns of miRNA in Mythimna separata Walker (Lepidotera: Noctuidae).Genes (Basel). 2025 Feb 19;16(2):234. doi: 10.3390/genes16020234. Genes (Basel). 2025. PMID: 40004562 Free PMC article.
-
MiR-2b-2-5p regulates lipid metabolism and reproduction by targeting CREB in Bactrocera dorsalis.RNA Biol. 2023 Jan;20(1):164-176. doi: 10.1080/15476286.2023.2204579. RNA Biol. 2023. PMID: 37092804 Free PMC article.
-
Spätzle Regulates Developmental and Immune Trade-Offs Induced by Bacillus thuringiensis Priming in Rhynchophorus ferrugineus.Insects. 2024 Nov 26;15(12):925. doi: 10.3390/insects15120925. Insects. 2024. PMID: 39769527 Free PMC article.
References
-
- Kaspi R, Mossinson S, Drezner T, Kamensky B, Yuval B. Effects of larval diet on development rates and reproductive maturation of male and female Mediterranean fruit flies. PHYSIOL ENTOMOL. 2002;27(1):29–38. doi: 10.1046/j.1365-3032.2001.00264.x - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

