Changes in Coagulation Testing During a National Shortage of Blue-Top Tubes
- PMID: 36197906
- PMCID: PMC9619712
- DOI: 10.1093/ajcp/aqac121
Changes in Coagulation Testing During a National Shortage of Blue-Top Tubes
Abstract
Objectives: Manufacturer recalls and altered supply chains during the coronavirus disease 2019 (COVID-19) pandemic caused a nationwide shortage of blue-top tubes (BTTs). Most non-point-of-care coagulation tests use these tubes, leaving laboratories and health care facilities in short supply. The Department of Pathology and Laboratory Medicine at Cedars-Sinai Medical Center implemented interventions to conserve supply without sacrificing patient safety.
Methods: In a retrospective quality improvement analysis, we examined coagulation testing and BTT utilization over the 3-month interval during which our interventions were applied. Our study assessed the interventions' effectiveness by evaluating changes in BTT utilization, coagulation testing volume, and patient impact.
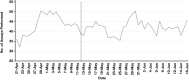
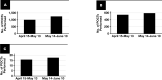
Results: Average daily use (ADU) of BTT before and after the intervention were 476 and 403, respectively-a 15.2% reduction. Notably, the Emergency Department had a reduction in ADU of 43.3%. Average daily volumes of coagulation assays performed decreased from 949 to 783-a 17.5% reduction. No adverse events from the Pharmacy Department were identified during the study period.
Conclusions: Interventions resulting in significant reductions were in divisions with effective management and supervision. Success in navigating the BTT shortage stemmed from timely announcements, action, and effective communication. Our recommendations established more effective coagulation assay utilization, decreased overall BTT use, and prevented patients with coagulopathic disorders from experiencing adverse consequences.
Keywords: Blue-top tubes; COVID-19; Laboratory stewardship; Shortage.
© The Author(s) 2022. Published by Oxford University Press on behalf of American Society for Clinical Pathology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




Comment in
-
Optimizing order entry presentation: a simple tool to help decrease redundancy in coagulation testing.Am J Clin Pathol. 2023 May 2;159(5):517-518. doi: 10.1093/ajcp/aqad020. Am J Clin Pathol. 2023. PMID: 36897263 No abstract available.
References
-
- Class 2 device recall VACUETTE blood collection tube 9NC coagulation sodium citrate 3.2. US Food and Drug Administration Web site. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=186712. Updated September 2, 2022. Accessed August 29, 2021.
-
- Sodium citrate blood specimen collection tube conservation strategies—letter to health care and laboratory personnel. US Food and Drug Administration Web site. https://www.fda.gov/medical-devices/letters-health-care-providers/sodium.... Updated January 19, 2022. Accessed September 4, 2021.
-
- Blue top tube recommendations. College of American Pathologists Web site. https://documents.cap.org/documents/cap-blue-top-tubes-recommendations.pdf. Published June 15, 2021. Accessed September 4, 2021.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

