A unique biomimetic modification endows polyetherketoneketone scaffold with osteoinductivity by activating cAMP/PKA signaling pathway
- PMID: 36197987
- PMCID: PMC9534509
- DOI: 10.1126/sciadv.abq7116
A unique biomimetic modification endows polyetherketoneketone scaffold with osteoinductivity by activating cAMP/PKA signaling pathway
Abstract
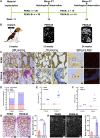
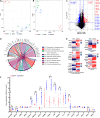
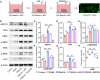
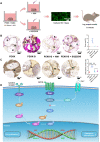
Osteoinductivity of a biomaterial scaffold can notably enhance the bone healing performance. In this study, we developed a biomimetic and hierarchically porous polyetherketoneketone (PEKK) scaffold with unique osteoinductivity using a combined surface treatment strategy of a sulfonated process and a nano bone-like apatite deposition. In a beagle intramuscular model, the scaffold induced bone formation ectopically after 12-week implantation. The better bone healing ability of the scaffold than the original PEKK was also confirmed in orthotopic sites. After culturing with bone marrow-derived mesenchymal stem cells (BMSCs), the scaffold induced osteogenic differentiation of BMSCs, and the new bone formation could be mainly depending on cell signaling through adenylate cyclase 9, which activates the cyclic adenosine monophosphate/protein kinase A signaling cascade pathways. The current work reports a new osteoinductive synthetic polymeric scaffold with its detailed molecular mechanism of action for bone repair and regeneration.
Figures


Similar articles
-
Mesenchymal stem cells seeded onto tissue-engineered osteoinductive scaffolds enhance the healing process of critical-sized radial bone defects in rat.Cell Tissue Res. 2018 Oct;374(1):63-81. doi: 10.1007/s00441-018-2837-7. Epub 2018 May 1. Cell Tissue Res. 2018. Retraction in: Cell Tissue Res. 2021 Oct;386(1):211. doi: 10.1007/s00441-021-03520-w. PMID: 29717356 Retracted.
-
Intermittent parathyroid hormone (1-34) application regulates cAMP-response element binding protein activity to promote the proliferation and osteogenic differentiation of bone mesenchymal stromal cells, via the cAMP/PKA signaling pathway.Exp Ther Med. 2016 Jun;11(6):2399-2406. doi: 10.3892/etm.2016.3177. Epub 2016 Mar 22. Exp Ther Med. 2016. PMID: 27284327 Free PMC article.
-
A silk fibroin/chitosan/nanohydroxyapatite biomimetic bone scaffold combined with autologous concentrated growth factor promotes the proliferation and osteogenic differentiation of BMSCs and repair of critical bone defects.Regen Ther. 2022 Sep 2;21:307-321. doi: 10.1016/j.reth.2022.08.006. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36110973 Free PMC article.
-
Suppressing mesenchymal stem cell hypertrophy and endochondral ossification in 3D cartilage regeneration with nanofibrous poly(l-lactic acid) scaffold and matrilin-3.Acta Biomater. 2018 Aug;76:29-38. doi: 10.1016/j.actbio.2018.06.027. Epub 2018 Jun 22. Acta Biomater. 2018. PMID: 29940371 Free PMC article.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
Cited by
-
Hydroxyapatite Scaffold and Bioactive Factor Combination as a Tool to Improve Osteogenesis, In Vitro and In Vivo Experiments Using Phage Display Technology.Int J Mol Sci. 2025 Jul 22;26(15):7040. doi: 10.3390/ijms26157040. Int J Mol Sci. 2025. PMID: 40806172 Free PMC article.
-
Biomineralized PEEK cages containing osteoinductive CaP bioceramics promote spinal fusion in goats.Bioact Mater. 2024 Nov 20;45:128-147. doi: 10.1016/j.bioactmat.2024.11.014. eCollection 2025 Mar. Bioact Mater. 2024. PMID: 40529190 Free PMC article.
-
Recent Advances in Peptide-Functionalized Hydrogels for Bone Tissue Engineering.ACS Biomater Sci Eng. 2025 Apr 14;11(4):1970-1989. doi: 10.1021/acsbiomaterials.4c02198. Epub 2025 Apr 3. ACS Biomater Sci Eng. 2025. PMID: 40178194 Review.
-
Treatment of large bone defects in load-bearing bone: traditional and novel bone grafts.J Zhejiang Univ Sci B. 2025 Apr 30;26(5):421-447. doi: 10.1631/jzus.B2300669. J Zhejiang Univ Sci B. 2025. PMID: 40436640 Free PMC article. Review.
-
Rapid fabrication of biomimetic PLGA microsphere incorporated with natural porcine dermal aECM for bone regeneration.Regen Biomater. 2024 Aug 26;11:rbae099. doi: 10.1093/rb/rbae099. eCollection 2024. Regen Biomater. 2024. PMID: 39463918 Free PMC article.
References
-
- Li J. J., Dunstan C. R., Entezari A., Li Q., Steck R., Saifzadeh S., Sadeghpour A., Field J. R., Akey A., Vielreicher M., Friedrich O., Roohani-Esfahani S. I., Zreiqat H., A novel bone substitute with high bioactivity, strength, and porosity for repairing large and load-bearing bone defects. Adv. Healthc. Mater. 8, e1801298 (2019). - PubMed
-
- Stevens M. M., Biomaterials for bone tissue engineering. Mater. Today 11, 18–25 (2008).
LinkOut - more resources
Full Text Sources

