Role of serum-free light chain assay for defining response and progression in immunoglobulin secretory multiple myeloma
- PMID: 36198076
- PMCID: PMC9828555
- DOI: 10.1002/ajh.26747
Role of serum-free light chain assay for defining response and progression in immunoglobulin secretory multiple myeloma
Abstract
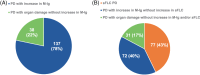
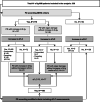
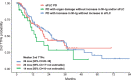
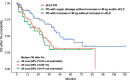
The International Myeloma Working Group (IMWG) guidelines recommend using electrophoresis and immunofixation to define response and progressive disease (PD) in immunoglobulin (Ig) secretory multiple myeloma (Ig-MM), whereas the role of serum-free light chain (sFLC) is controversial. We retrospectively analyzed the value of adding sFLC assays in the definition of response and PD according to IMWG criteria in 339 Ig-MM patients treated with a first-line novel agent-based therapy (median follow-up 54 months). sFLC PD was defined according to conventional criteria plus increased sFLC levels, or sFLC escape (sFLCe); progression/sFLCe-free survival (ePFS) was the time from the start of treatment to the date of first PD or sFLCe, or death; overall survival after PD/sFLCe (OS after Pe) was the time from first PD or sFLCe to the date of death. 148 (44%) patients achieved a complete response and 198 (60%) a normal sFLC ratio (sFLCR). sFLCR normalization was an independent prognostic factor for extended PFS (HR = 0.46, p = 0.001) and OS (HR = 0.47, p = 0.006) by multivariable analysis. 175 (52%) patients experienced PD according to the IMWG criteria, whereas 180 (53%) experienced PD or sFLCe. Overall, a sFLCe was observed in 31 (9%) patients. Median PFS and ePFS were both equal to 36 (95% CI = 32-42, and 32-40, respectively) months. sFLC PD adversely affected the OS after Pe compared to PD with increasing monoclonal Ig only (HR = 0.52, p = 0.012). Our results support the inclusion of the sFLC assay for defining response and PD in Ig-MM.
© 2022 The Authors. American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
Paola Tacchetti has received Honoraria from Amgen, Bristol‐Myers Squibb/Celgene, Janssen, Takeda, AbbVie, Sanofi, GlaxoSmithKline, and Oncopeptides; Serena Rocchi receives Honoraria from Amgen, GlaxoSmithKline, and Janssen; Elena Zamagni has received honoraria from Janssen, Bristol‐Myers Squibb, Amgen, Takeda; Ilaria Rizzello has received Honoraria from Amgen, GlaxoSmithKline, and Sanofi; Lucia Pantani has received Honoraria from Janssen and Amgen; Katia Mancuso has received Honoraria from Celgene, Takeda, Amgen, Sanofi and Janssen; Michele Cavo has received Honoraria from Janssen, Celgene, Amgen, Bristol‐Myers Squibb, Takeda, AbbVie, Sanofi, Adaptive Biotechnologies, and is a member of Janssen's and Celgene's Speaker's Bureau; Simona Barbato, Gabriella De Cicco, Alessio Fusco, Luca Dozza, Margherita Ursi, Emanuele Favero, Carolina Terragna and Nicoletta Testoni declare no potential conflicts of interest.
Figures
References
-
- Bradwell AR, Carr‐Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47(4):673‐680. - PubMed
-
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538‐e548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
