Heterogeneous M-N-C Catalysts for Aerobic Oxidation Reactions: Lessons from Oxygen Reduction Electrocatalysts
- PMID: 36198176
- PMCID: PMC10073352
- DOI: 10.1021/acs.chemrev.2c00424
Heterogeneous M-N-C Catalysts for Aerobic Oxidation Reactions: Lessons from Oxygen Reduction Electrocatalysts
Abstract
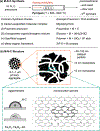
Nonprecious metal heterogeneous catalysts composed of first-row transition metals incorporated into nitrogen-doped carbon matrices (M-N-Cs) have been studied for decades as leading alternatives to Pt for the electrocatalytic O2 reduction reaction (ORR). More recently, similar M-N-C catalysts have been shown to catalyze the aerobic oxidation of organic molecules. This Focus Review highlights mechanistic similarities and distinctions between these two reaction classes and then surveys the aerobic oxidation reactions catalyzed by M-N-Cs. As the active-site structures and kinetic properties of M-N-C aerobic oxidation catalysts have not been extensively studied, the array of tools and methods used to characterize ORR catalysts are presented with the goal of supporting further advances in the field of aerobic oxidation.
Figures
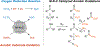






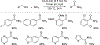




References
-
- Cui X; Li W; Ryabchuk P; Junge K; Beller M Bridging Homogeneous and Heterogeneous Catalysis by Heterogeneous Single-Metal-Site Catalysts. Nat. Catal 2018, 1, 385.
-
- Kaiser SK; Chen Z; Faust Akl D; Mitchell S; Pérez-Ramírez J Single-Atom Catalysts across the Periodic Table. Chem. Rev 2020, 120, 11703–11809. - PubMed
-
- Bagotzky VS; Tarasevich MR; Radyushkina KA; Levina OA; Andrusyova SI Electrocatalysis of the Oxygen Reduction Process on Metal Chelates in Acid Electrolyte. J. Power Sources 1978, 2, 233–240.
-
- van Veen JAR; van Baar JF; Kroese KJ Effect of Heat Treatment on the Performance of Carbon-Supported Transition-Metal Chelates in the Electrochemical Reduction of Oxygen. J. Chem. Soc., Faraday Trans January 1981, 77, 2827–2843.
-
- Scherson DA; Gupta SL; Fierro C; Yeager EB; Kordesch ME; Eldridge J; Hoffman RW; Blue J Cobalt Tetramethoxyphenyl Porphyrin—Emission Mossbauer Spectroscopy and O2 Reduction Electrochemical Studies. Electrochim. Acta 1983, 28, 1205–1209.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

