Molecular basis for processing of topoisomerase 1-triggered DNA damage by Apn2/APE2
- PMID: 36198268
- PMCID: PMC9732815
- DOI: 10.1016/j.celrep.2022.111448
Molecular basis for processing of topoisomerase 1-triggered DNA damage by Apn2/APE2
Abstract
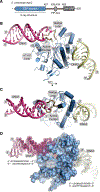
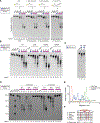
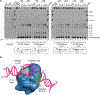
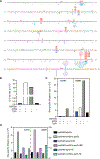
Topoisomerase 1 (Top1) incises DNA containing ribonucleotides to generate complex DNA lesions that are resolved by APE2 (Apn2 in yeast). How Apn2 engages and processes this DNA damage is unclear. Here, we report X-ray crystal structures and biochemical analysis of Apn2-DNA complexes to demonstrate how Apn2 frays and cleaves 3' DNA termini via a wedging mechanism that facilitates 1-6 nucleotide endonucleolytic cleavages. APN2 deletion and DNA-wedge mutant Saccharomyces cerevisiae strains display mutator phenotypes, cell growth defects, and sensitivity to genotoxic stress in a ribonucleotide excision repair (RER)-defective background harboring a high density of Top1-incised ribonucleotides. Our data implicate a wedge-and-cut mechanism underpinning the broad-specificity Apn2 nuclease activity that mitigates mutagenic and genome instability phenotypes caused by Top1 incision at genomic ribonucleotides incorporated by DNA polymerase epsilon.
Keywords: APE2; Apn2; CP: molecular biology; DNA damage; DNA repair; DNA replication; S. cerevisiae; Top1; X-ray crystallography; ribonucleotide.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Adams PD, Afonine PV, Bunkó czi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr 66, 213–221. 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Álvarez-Quilón A, Wojtaszek JL, Mathieu MC, Patel T, Appel CD, Hustedt N, Rossi SE, Wallace BD, Setiaputra D, Adam S, et al. (2020). Endogenous DNA 3’ blocks are vulnerabilities for BRCA1 and BRCA2 deficiency and are reversed by the APE2 nuclease. Mol. Cell 78, 1152–1165.e8. 10.1016/j.molcel.2020.05.021. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

