An efficient approach for SARS-CoV-2 monoclonal antibody production via modified mRNA-LNP immunization
- PMID: 36198358
- PMCID: PMC9526872
- DOI: 10.1016/j.ijpharm.2022.122256
An efficient approach for SARS-CoV-2 monoclonal antibody production via modified mRNA-LNP immunization
Abstract
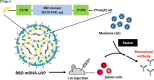
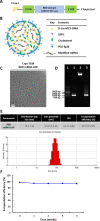
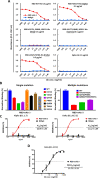
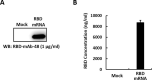
Throughout the COVID-19 pandemic, many prophylactic and therapeutic drugs have been evaluated and introduced. Among these treatments, monoclonal antibodies (mAbs) that bind to and neutralize SARS-CoV-2 virus have been applied as complementary and alternative treatments to vaccines. Although different methodologies have been utilized to produce mAbs, traditional hybridoma fusion technology is still commonly used for this purpose due to its unmatched performance record. In this study, we coupled the hybridoma fusion strategy with mRNA-lipid nanoparticle (LNP) immunization. This time-saving approach can circumvent biological and technical hurdles, such as difficult-to-express membrane proteins, antigen instability, and the lack of posttranslational modifications on recombinant antigens. We used mRNA-LNP immunization and hybridoma fusion technology to generate mAbs against the receptor binding domain (RBD) of SARS-CoV-2 spike (S) protein. Compared with traditional protein-based immunization approaches, inoculation of mice with RBD mRNA-LNP induced higher titers of serum antibodies and markedly increased serum neutralizing activity. The mAbs we obtained can bind to SARS-CoV-2 RBDs from several variants. Notably, RBD-mAb-3 displayed particularly high binding affinities and neutralizing potencies against both Alpha and Delta variants. In addition to introducing specific mAbs against SARS-CoV-2, our data generally demonstrate that mRNA-LNP immunization may be useful to quickly generate highly functional mAbs against emerging infectious diseases.
Keywords: COVID-19; Lipid nanoparticle (LNP); Modified mRNA; Monoclonal antibodies; SARS-CoV-2.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Chapman A.P., Tang X., Lee J.R., Chida A., Mercer K., Wharton R.E., Kainulainen M., Harcourt J.L., Martines R.B., Schroeder M., Zhao L., Bryksin A., Zhou B., Bergeron E., Bollweg B.C., Tamin A., Thornburg N., Wentworth D.E., Petway D., Bagarozzi D.A., Jr, Finn M.G., Goldstein J.M. Rapid development of neutralizing and diagnostic SARS-COV-2 mouse monoclonal antibodies. Sci. Rep. 2021;11:9682. - PMC - PubMed
-
- Chiarella P., Fazio V.M. Mouse monoclonal antibodies in biological research: strategies for high-throughput production. Biotechnol. Lett. 2008;30(8):1303–1310. - PubMed
-
- Du W., Hurdiss D.L., Drabek D., Mykytyn A.Z., Kaiser F.K., González-Hernández M., Muñoz-Santos D., Lamers M.M., van Haperen R., Li W., Drulyte I., Wang C., Sola I., Armando F., Beythien G., Ciurkiewicz M., Baumgärtner W., Guilfoyle K., Smits T., van der Lee J., van Kuppeveld F.J.M., van Amerongen G., Haagmans B.L., Enjuanes L., Osterhaus A.D.M.E., Grosveld F., Bosch B.J. An ACE2-blocking antibody confers broad neutralization and protection against Omicron and other SARS-CoV-2 variants of concern. Sci. Immunol. 2022;26:eabp9312. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

