E. coli 6S RNA complexed to RNA polymerase maintains product RNA synthesis at low cellular ATP levels by initiation with noncanonical initiator nucleotides
- PMID: 36198425
- PMCID: PMC9670815
- DOI: 10.1261/rna.079356.122
E. coli 6S RNA complexed to RNA polymerase maintains product RNA synthesis at low cellular ATP levels by initiation with noncanonical initiator nucleotides
Abstract
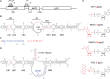
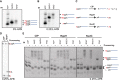
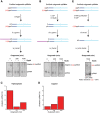
The E. coli 6S RNA is an RNA polymerase (RNAP) inhibitor that competes with σ70-dependent DNA promoters for binding to RNAP holoenzyme (RNAP:σ70). The 6S RNA when bound is then used as a template to synthesize a short product RNA (pRNA; usually 13-nt-long). This pRNA changes the 6S RNA structure, triggering the 6S RNA:pRNA complex to release and allowing DNA-dependent housekeeping gene expression to resume. In high nutrient conditions, 6S RNA turnover is extremely rapid but becomes very slow in low nutrient environments. This leads to a large accumulation of inhibited RNAP:σ70 in stationary phase. As pRNA initiates synthesis with ATP, we and others have proposed that the 6S RNA release rate strongly depends on ATP levels as a proxy for sensing the cellular metabolic state. By purifying endogenous 6S RNA:pRNA complexes using RNA Mango and using reverse transcriptase to generate pRNA-cDNA chimeras, we demonstrate that 6S RNA:pRNA formation can be simultaneous with 6S RNA 5' maturation. More importantly, we find a dramatic accumulation of capped pRNAs during stationary phase. This indicates that ATP levels in stationary phase are low enough for noncanonical initiator nucleotides (NCINs) such as NAD+ and NADH to initiate pRNA synthesis. In vitro, mutation of the conserved 6S RNA template sequence immediately upstream of the pRNA transcriptional start site can increase or decrease the pRNA capping efficiency, suggesting that evolution has tuned the biological 6S RNA sequence for an optimal capping rate. NCIN-initiated pRNA synthesis may therefore be essential for cell viability in low nutrient conditions.
Keywords: 6S RNA; ATP; NAD(H); RNA Mango; exponential phase; pRNA; stationary phase.
© 2022 Bonar et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
