Characterization and corroboration of safety signals identified from the US Food and Drug Administration Adverse Event Reporting System, 2008-19: cross sectional study
- PMID: 36198428
- PMCID: PMC9533298
- DOI: 10.1136/bmj-2022-071752
Characterization and corroboration of safety signals identified from the US Food and Drug Administration Adverse Event Reporting System, 2008-19: cross sectional study
Abstract
Objective: To characterize potential drug safety signals identified from the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), from 2008 to 2019, to determine how often these signals resulted in regulatory action by the FDA and whether these actions were corroborated by published research findings or public assessments by the Sentinel Initiative.
Design: Cross sectional study.
Setting: USA.
Population: Safety signals identified from the FAERS and publicly reported by the FDA between 2008 and 2019; and review of the relevant literature published before and after safety signals were reported in 2014-15. Literature searches were performed in November 2019, Sentinel Initiative assessments were searched in December 2021, and data analysis was finalized in December 2021.
Main outcome measures: Safety signals and resulting regulatory actions; number and characteristics of published studies, including corroboration of regulatory action as evidenced by significant associations (or no associations) between the drug related to the signal and the adverse event.
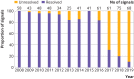
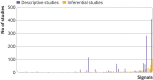
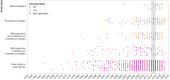
Results: From 2008 to 2019, 603 potential safety signals identified from the FAERS were reported by the FDA (median 48 annually, interquartile range 41-61), of which 413 (68.5%) were resolved as of December 2021 (372 of 399 (93.2%) signals ≥3 years old were resolved). Among the resolved safety signals, 91 (22.0%) led to no regulatory action and 322 (78.0%) resulted in regulatory action, including 319 (77.2%) changes to drug labeling and 59 (14.3%) drug safety communications or other public communications from the FDA. For a subset of 82 potential safety signals reported in 2014-15, a literature search identified 1712 relevant publications; 1201 (70.2%) were case reports or case series. Among these 82 safety signals, 76 (92.7%) were resolved, of which relevant published research was identified for 57 (75.0%) signals and relevant Sentinel Initiative assessments for four (5.3%) signals. Regulatory actions by the FDA were corroborated by at least one relevant published research study for 17 of the 57 (29.8%) resolved safety signals; none of the relevant Sentinel Initiative assessments corroborated FDA regulatory action.
Conclusions: Most potential safety signals identified from the FAERS led to regulatory action by the FDA. Only a third of regulatory actions were corroborated by published research, however, and none by public assessments from the Sentinel Initiative. These findings suggest that either the FDA is taking regulatory actions based on evidence not made publicly available or more comprehensive safety evaluations might be needed when potential safety signals are identified.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organization for the submitted work; MMD reports receiving grants from the Yale-Mayo Clinic Center of Excellence in Regulatory Science and Innovation (CERSI) Scholars outside the submitted work; RR reports research support through Yale University from Arnold Ventures and the Stavros Niarchos Foundation and was an employee of the Veterans Health Administration, but the views expressed in this article are those of the authors and do not necessarily reflect those of the US Department of Veteran Affairs or the US government; XS is supported by the China Scholarship Council; JDW is supported by the FDA, Johnson and Johnson through Yale University, Arnold Ventures, and the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award 1K01AA028258; JDW serves as a consultant for Hagens Berman Sobol Shapiro LLP and Dugan Law Firm APLC.111; JSR is the US outreach and associate research editor at The BMJ and currently receives research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the Medical Device Innovation Consortium as part of the National Evaluation System for Health Technology (NEST), from the Food and Drug Administration for the Yale-Mayo Clinic Center of Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), from the Agency for Healthcare Research and Quality (R01HS022882), from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R01HS025164, R01HL144644), and from Arnold Ventures; JSR is an expert witness at the request of Relator’s attorneys, the Greene Law Firm, in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen Inc; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Radical transparency in post-market oversight of medicine safety.BMJ. 2022 Oct 5;379:o2275. doi: 10.1136/bmj.o2275. BMJ. 2022. PMID: 36198408 No abstract available.
-
Transparency and robustness of safety signals.BMJ. 2022 Nov 3;379:o2588. doi: 10.1136/bmj.o2588. BMJ. 2022. PMID: 36328354 No abstract available.
References
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998;279:1200-5. - PubMed
-
- US Food and Drug Administration Center for Drug Evaluation and Research. Office of Surveillance and Epidemiology 2021 Annual Report. https://www.fda.gov/media/157388/download. Published 2022. Accessed July 29, 2022.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
