3D-printed microrobots from design to translation
- PMID: 36198675
- PMCID: PMC9534872
- DOI: 10.1038/s41467-022-33409-3
3D-printed microrobots from design to translation
Abstract
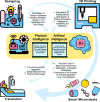
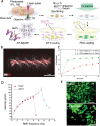
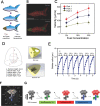
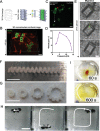
Microrobots have attracted the attention of scientists owing to their unique features to accomplish tasks in hard-to-reach sites in the human body. Microrobots can be precisely actuated and maneuvered individually or in a swarm for cargo delivery, sampling, surgery, and imaging applications. In addition, microrobots have found applications in the environmental sector (e.g., water treatment). Besides, recent advancements of three-dimensional (3D) printers have enabled the high-resolution fabrication of microrobots with a faster design-production turnaround time for users with limited micromanufacturing skills. Here, the latest end applications of 3D printed microrobots are reviewed (ranging from environmental to biomedical applications) along with a brief discussion over the feasible actuation methods (e.g., on- and off-board), and practical 3D printing technologies for microrobot fabrication. In addition, as a future perspective, we discussed the potential advantages of integration of microrobots with smart materials, and conceivable benefits of implementation of artificial intelligence (AI), as well as physical intelligence (PI). Moreover, in order to facilitate bench-to-bedside translation of microrobots, current challenges impeding clinical translation of microrobots are elaborated, including entry obstacles (e.g., immune system attacks) and cumbersome standard test procedures to ensure biocompatibility.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Bogue, R. Miniature and microrobots: a review of recent developments. Ind. Robot42, 98–102 2015).
-
- Donald BR, Levey CG, Paprotny I. Planar microassembly by parallel actuation of MEMS microrobots. J. Microelectromech. Syst. 2008;17:789–808. doi: 10.1109/JMEMS.2008.924251. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

