Deletion of skeletal muscle Akt1/2 causes osteosarcopenia and reduces lifespan in mice
- PMID: 36198696
- PMCID: PMC9535008
- DOI: 10.1038/s41467-022-33008-2
Deletion of skeletal muscle Akt1/2 causes osteosarcopenia and reduces lifespan in mice
Abstract
Aging is considered to be accelerated by insulin signaling in lower organisms, but it remained unclear whether this could hold true for mammals. Here we show that mice with skeletal muscle-specific double knockout of Akt1/2, key downstream molecules of insulin signaling, serve as a model of premature sarcopenia with insulin resistance. The knockout mice exhibit a progressive reduction in skeletal muscle mass, impairment of motor function and systemic insulin sensitivity. They also show osteopenia, and reduced lifespan largely due to death from debilitation on normal chow and death from tumor on high-fat diet. These phenotypes are almost reversed by additional knocking out of Foxo1/4, but only partially by additional knocking out of Tsc2 to activate the mTOR pathway. Overall, our data suggest that, unlike in lower organisms, suppression of Akt activity in skeletal muscle of mammals associated with insulin resistance and aging could accelerate osteosarcopenia and consequently reduce lifespan.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

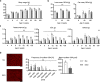


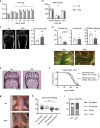
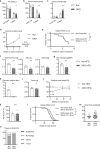
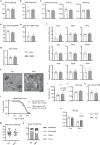

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

