Influence of Ru on structure and corrosion behavior of passive film on Ti-6Al-4V alloy in oil and gas exploration conditions
- PMID: 36198740
- PMCID: PMC9535010
- DOI: 10.1038/s41598-022-21047-0
Influence of Ru on structure and corrosion behavior of passive film on Ti-6Al-4V alloy in oil and gas exploration conditions
Abstract
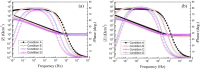
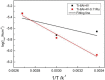
In order to investigate the influence of minor Ru on the electrochemical behaviour and structural characteristics of passive films on the surface of Ti-6Al-4V alloys under various oil and gas exploration conditions, electrochemical techniques, X-ray photoelectron spectroscopy (XPS), scanning electron microscope (SEM) and corrosion simulation tests were carried out. The results revealed that the oil and gas exploration conditions had a serious impact on the electrochemical behaviour and corrosion resistance of the tested alloys. The passivation film resistance and corrosion potential of the tested titanium alloys were significantly reduced with increasing acidity and temperature. With the addition of minor ruthenium, the potential of the passive film on the Ti-6Al-4V-0.11Ru alloy surface increased because of the high surface potential of the ruthenium element. The contents of metallic ruthenium and tetravalent titanium oxide TiO2 in the surface film of the Ti-6Al-4V-0.11Ru alloy both increased with increasing temperature, which led to increase the thickness, stability, corrosion resistance and repairability of the passive film on the surface of the Ti-6Al-4V-0.11Ru alloy being better than those qualities of Ti-6Al-4V. These results were also confirmed by corrosion simulation tests.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
















References
-
- Shadravan A, Amani M. What petroleum engineers and geoscientists should know about high pressure high temperature wells environment. Energy Sci. Technol. 2012;4(2):36.
-
- Ye DS, Ren Y, Guan B, et al. Prediction on wellhead outflow performance by dynamic friction coefficient of gas wells. Nat. Gas. Ind. 2009;29(3):77–82.
-
- Gu T, Huo SQ, Li F, et al. Research and application of anti-corrosion technology in sour gas fields. Chem. Eng. Oil Gas. 2008;37(Supplement):63–72.
-
- Zhang SC, Zhang BM, Li BL, et al. History of hydrocarbon accumulations spanning important tectonic phases in marine sedimentary basins of China: Taking the Tarim Basin as an example. Pet. Explor. Dev. 2011;38(2):1–15.
-
- Schutz RW, Watkins HB. Recent developments in titanium alloy application in the energy industry. Mater. Sci. Eng. A. 1998;243:305–315. doi: 10.1016/S0921-5093(97)00819-8. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

