NMR-guided directed evolution
- PMID: 36198791
- PMCID: PMC10116341
- DOI: 10.1038/s41586-022-05278-9
NMR-guided directed evolution
Abstract
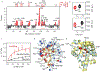
Directed evolution is a powerful tool for improving existing properties and imparting completely new functionalities to proteins1-4. Nonetheless, its potential in even small proteins is inherently limited by the astronomical number of possible amino acid sequences. Sampling the complete sequence space of a 100-residue protein would require testing of 20100 combinations, which is beyond any existing experimental approach. In practice, selective modification of relatively few residues is sufficient for efficient improvement, functional enhancement and repurposing of existing proteins5. Moreover, computational methods have been developed to predict the locations and, in certain cases, identities of potentially productive mutations6-9. Importantly, all current approaches for prediction of hot spots and productive mutations rely heavily on structural information and/or bioinformatics, which is not always available for proteins of interest. Moreover, they offer a limited ability to identify beneficial mutations far from the active site, even though such changes may markedly improve the catalytic properties of an enzyme10. Machine learning methods have recently showed promise in predicting productive mutations11, but they frequently require large, high-quality training datasets, which are difficult to obtain in directed evolution experiments. Here we show that mutagenic hot spots in enzymes can be identified using NMR spectroscopy. In a proof-of-concept study, we converted myoglobin, a non-enzymatic oxygen storage protein, into a highly efficient Kemp eliminase using only three mutations. The observed levels of catalytic efficiency exceed those of proteins designed using current approaches and are similar with those of natural enzymes for the reactions that they are evolved to catalyse. Given the simplicity of this experimental approach, which requires no a priori structural or bioinformatic knowledge, we expect it to be widely applicable and to enable the full potential of directed enzyme evolution.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures






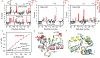
References
-
- Bornscheuer UT et al. Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012). - PubMed
-
- Reetz MT Laboratory evolution of stereoselective enzymes: a prolific source of catalysts for asymmetric reactions. Angew. Chem. Int. Ed. Engl. 50, 138–174 (2011). - PubMed
-
- Denard CA, Ren H & Zhao H Improving and repurposing biocatalysts via directed evolution. Curr. Opin. Chem. Biol. 25, 55–64 (2015). - PubMed
-
- Chen K & Arnold FH Engineering new catalytic activities in enzymes. Nat. Catal 3, 203–213 (2020).
-
- Reetz MT, Wilensek S, Zha D & Jaeger KE Directed evolution of an enantioselective enzyme through combinatorial multiple-cassette mutagenesis. Angew. Chem. Int. Ed. Engl. 40, 3589–3591 (2001). - PubMed
Methods references
-
- Casey ML, Kemp DS, Paul KG & Cox DD Physical organic chemistry of benzisoxazoles. I. Mechanism of base-catalyzed decomposition of benzisoxazoles. J. Org. Chem. 38, 2294–2301 (1973).
-
- Berry EA & Trumpower BL Simultaneous determination of hemes a, hemes b, and hemes c from pyridine hemochrome spectra. Anal. Biochem. 161, 1–15 (1987). - PubMed
-
- Barik S Megaprimer PCR. in PCR Cloning Protocols (eds. Chen B-Y & Janes H) 189–196 (Humana Press, Totowa, 2002).
-
- Delaglio F et al. NMRPipe - a multidimensional spectral processing system based on UNIX Pipes. J. Biomol. NMR 6, 277–293 (1995). - PubMed
-
- Vranken WF et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

