Nuclear-embedded mitochondrial DNA sequences in 66,083 human genomes
- PMID: 36198798
- PMCID: PMC9630118
- DOI: 10.1038/s41586-022-05288-7
Nuclear-embedded mitochondrial DNA sequences in 66,083 human genomes
Abstract
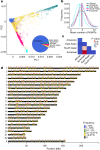
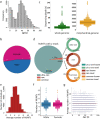
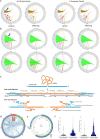
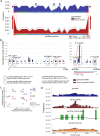
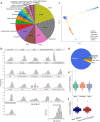
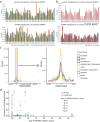
DNA transfer from cytoplasmic organelles to the cell nucleus is a legacy of the endosymbiotic event-the majority of nuclear-mitochondrial segments (NUMTs) are thought to be ancient, preceding human speciation1-3. Here we analyse whole-genome sequences from 66,083 people-including 12,509 people with cancer-and demonstrate the ongoing transfer of mitochondrial DNA into the nucleus, contributing to a complex NUMT landscape. More than 99% of individuals had at least one of 1,637 different NUMTs, with 1 in 8 individuals having an ultra-rare NUMT that is present in less than 0.1% of the population. More than 90% of the extant NUMTs that we evaluated inserted into the nuclear genome after humans diverged from apes. Once embedded, the sequences were no longer under the evolutionary constraint seen within the mitochondrion, and NUMT-specific mutations had a different mutational signature to mitochondrial DNA. De novo NUMTs were observed in the germline once in every 104 births and once in every 103 cancers. NUMTs preferentially involved non-coding mitochondrial DNA, linking transcription and replication to their origin, with nuclear insertion involving multiple mechanisms including double-strand break repair associated with PR domain zinc-finger protein 9 (PRDM9) binding. The frequency of tumour-specific NUMTs differed between cancers, including a probably causal insertion in a myxoid liposarcoma. We found evidence of selection against NUMTs on the basis of size and genomic location, shaping a highly heterogenous and dynamic human NUMT landscape.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

