HSP90β promotes osteoclastogenesis by dual-activation of cholesterol synthesis and NF-κB signaling
- PMID: 36198833
- PMCID: PMC9984383
- DOI: 10.1038/s41418-022-01071-3
HSP90β promotes osteoclastogenesis by dual-activation of cholesterol synthesis and NF-κB signaling
Abstract
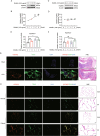
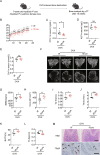
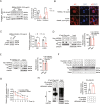
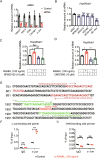
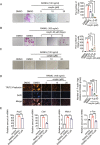
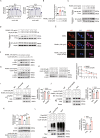
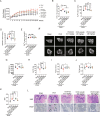
Heat shock protein 90β (Hsp90β, encoded by Hsp90ab1 gene) is the most abundant proteins in the cells and contributes to variety of biological processes including metabolism, cell growth and neural functions. However, genetic evidences showing Hsp90β in vivo functions using tissue specific knockout mice are still lacking. Here, we showed that Hsp90β exerted paralogue-specific role in osteoclastogenesis. Using myeloid-specific Hsp90ab1 knockout mice, we provided the first genetic evidence showing the in vivo function of Hsp90β. Hsp90β binds to Ikkβ and reduces its ubiquitylation and proteasomal degradation, thus leading to activated NF-κB signaling. Meanwhile, Hsp90β increases cholesterol biosynthesis by activating Srebp2. Both pathways promote osteoclastogenic genes expression. Genetic deletion of Hsp90ab1 in osteoclast or pharmacological inhibition of Hsp90β alleviates bone loss in ovariectomy-induced mice. Therefore, Hsp90β is a promising druggable target for the treatment of osteoporosis.
© 2022. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

