Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation
- PMID: 36198876
- PMCID: PMC9700722
- DOI: 10.1038/s41386-022-01453-8
Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation
Abstract
Transcranial magnetic stimulation (TMS) is a non-invasive technique for focal brain stimulation based on electromagnetic induction where a fluctuating magnetic field induces a small intracranial electric current in the brain. For more than 35 years, TMS has shown promise in the diagnosis and treatment of neurological and psychiatric disorders in adults. In this review, we provide a brief introduction to the TMS technique with a focus on repetitive TMS (rTMS) protocols, particularly theta-burst stimulation (TBS), and relevant rTMS-derived metrics of brain plasticity. We then discuss the TMS-EEG technique, the use of neuronavigation in TMS, the neural substrate of TBS measures of plasticity, the inter- and intraindividual variability of those measures, effects of age and genetic factors on TBS aftereffects, and then summarize alterations of TMS-TBS measures of plasticity in major neurological and psychiatric disorders including autism spectrum disorder, schizophrenia, depression, traumatic brain injury, Alzheimer's disease, and diabetes. Finally, we discuss the translational studies of TMS-TBS measures of plasticity and their therapeutic implications.
© 2022. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
AJ is an employee of Linus Health. APL is a cofounder of Linus Health and TI Solutions AG; serves on the scientific advisory boards for Starlab Neuroscience, Magstim Inc., and MedRhythms; and is listed as an inventor on several issued and pending patents on the real-time integration of non-invasive brain stimulation with electroencephalography and magnetic resonance imaging. AR is a founder and advisor for Neuromotion and PrevEP, serves on the medical advisory board or has consulted for Cavion, Epihunter, Gamify, Neural Dynamics, NeuroRex, Otsuka, Roche, and is listed as an inventor on a patent related to integration of TMS and EEG. The authors declare no competing interests.
Figures



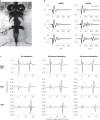
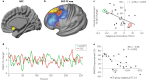

References
-
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. - PubMed
-
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;325:1106–7. - PubMed
-
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–65. - PubMed
-
- Barker AT. An introduction to the basic principles of magnetic nerve stimulation. J Clin Neurophysiol. 1991;8:26–37. - PubMed
-
- Barker AT. Transcranial magnetic stimulation—past, present and future. Brain Stimulation. 2017;10:540.

