Progress in xenotransplantation: overcoming immune barriers
- PMID: 36198911
- PMCID: PMC9671854
- DOI: 10.1038/s41581-022-00624-6
Progress in xenotransplantation: overcoming immune barriers
Abstract
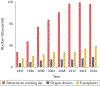
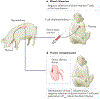
A major limitation of organ allotransplantation is the insufficient supply of donor organs. Consequently, thousands of patients die every year while waiting for a transplant. Progress in xenotransplantation that has permitted pig organ graft survivals of years in non-human primates has led to renewed excitement about the potential of this approach to alleviate the organ shortage. In 2022, the first pig-to-human heart transplant was performed on a compassionate use basis, and xenotransplantation experiments using pig kidneys in deceased human recipients provided encouraging data. Many advances in xenotransplantation have resulted from improvements in the ability to genetically modify pigs using CRISPR-Cas9 and other methodologies. Gene editing has the capacity to generate pig organs that more closely resemble those of humans and are hence more physiologically compatible and less prone to rejection. Despite such modifications, immune responses to xenografts remain powerful and multi-faceted, involving innate immune components that do not attack allografts. Thus, the induction of innate and adaptive immune tolerance to prevent rejection while preserving the capacity of the immune system to protect the recipient and the graft from infection is desirable to enable clinical xenotransplantation.
© 2022. Springer Nature Limited.
Figures


References
-
- OPTN. http://optn.transplant.hrsa.gov(2022).
-
- Kang HW, Yoo JJ & Atala A Bioprinted scaffolds for cartilage tissue engineering. Methods Mol. Biol 1340, 161–169 (2015). - PubMed
-
- Gilpin SE & Ott HC Using nature’s platform to engineer bio-artificial lungs. Ann. Am. Thorac. Soc 12, S45–S49 (2015). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

