The persistence of anti-Spike antibodies following two SARS-CoV-2 vaccine doses in patients on immunosuppressive therapy compared to healthy controls-a prospective cohort study
- PMID: 36199139
- PMCID: PMC9534475
- DOI: 10.1186/s12916-022-02587-8
The persistence of anti-Spike antibodies following two SARS-CoV-2 vaccine doses in patients on immunosuppressive therapy compared to healthy controls-a prospective cohort study
Abstract
Background: The durability of vaccine-induced humoral immunity against SARS-CoV-2 in patients with immune-mediated inflammatory diseases (IMIDs) on immunosuppressive therapy is not known. The aim of this study was to compare the persistence of anti-Spike antibodies following two-dose SARS-CoV-2 vaccination between IMID patients and healthy controls and to identify factors associated with antibody decline.
Methods: IMID patients on immunosuppressive medication enrolled in the prospective observational Nor-vaC study were included. Participants received two-dose SARS-CoV-2 vaccination. Serum collected at two time points following vaccination (first assessment within 6-48 days, second within 49-123 days) were analyzed for antibodies binding the receptor-binding domain (RBD) of the SARS-CoV-2 Spike protein. Multivariable regression models estimated percent reduction in anti-RBD over 30 days and factors associated with reduction.
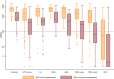
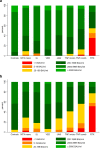
Results: A total of 1108 patients (403 rheumatoid arthritis, 195 psoriatic arthritis, 195 spondyloarthritis, 124 ulcerative colitis, 191 Crohn's disease) and 134 controls provided blood samples within the defined intervals (median 19 days [IQR 15-24] and 97 days [87-105] after second vaccine dose). Antibody levels were lower in patients compared to controls at both time points, with median anti-RBD 2806 BAU/ml [IQR 1018-6068] in patients and 6187 BAU/ml [4105-7496] in controls (p<0.001) at first assessment, and 608 BAU/ml [IQR 58-1053] in patients and 1520 BAU/ml [979-3766] in controls (p<0.001) at second assessment. At second assessment, low anti-RBD antibody levels (defined as <200 BAU/ml) were found in 449 (41%) patients, and 6 (5%) controls (p<0.001). The change was - 83% in patients and - 66% in controls (p<0.001). Patients had a greater estimated 30 days percent reduction in anti-RBD levels compared to controls - 4.9 (95% CI - 7.4 to - 2.4), (p<0.05). Among therapies, mono- or combination treatment with tumor necrosis factor inhibitors was associated with the greatest decline.
Conclusions: Within 4 months after vaccination, antibody levels declined considerably in both IMID patients and controls. Patients had lower initial antibody levels and a more pronounced decline compared to healthy controls and were therefore more likely to decline to low antibody levels. These results support that IMID patients need additional vaccine doses at an earlier stage than healthy individuals.
Keywords: COVID-19; Inflammatory bowel disease; Rheumatic diseases; SARS-CoV-2 vaccine; Serologic response.
© 2022. The Author(s).
Conflict of interest statement
TKK reports grants from AbbVie, Amgen, BMS, MSD, Novartis, Pfizer, UCB, consulting fees from AbbVie, Amgen, Biogen, Celltrion, Eli Lilly, Gilead, Mylan, Novartis, Pfizer, Sandoz, Sanofi, speakers bureaus Amgen, Celltrion, Egis, Evapharma, Ewopharma, Hikma, Oktal, Sandoz, Sanofi. JJ reports grants from Abbvie, Pharmacosmos, Ferring, consulting fees from Abbvie, Boerhinger Ingelheim, BMS, Celltrion, Ferring, Glihead, Janssen Cilag, MSD, Napp Pharma, Novartis, Orion Pharma, Pfeizer, Pharmacosmos, Takeda, Sandoz, Unimedic Pharma, speakers bureaus Abbvie, Astro Pharma, Boerhinger Ingelheim, BMS, Celltrion, Ferring, Glihead, Hikma, Janssen Cilag, Meda, MSD, Napp Pharma, Novartis, Oriuon Pharma, Pfeizer, Pharmacosmos, Roche, Takeda, Sandoz. LAM reports funding from KG Jebsen foundation, support for infrastructure and biobanking from the University of Oslo and Oslo University Hospital, grants from the Coalition of Epidemic Preparedness Innovations CEPI, speakers bureaus Novartis, Cellgene. JTV reports grant from the Coalition of Epidemic Preparedness Innovations (CEPI). GG reports consulting fees from the Norwegian System of Compensation to Patients, AstraZeneca, speakers bureaus Bayer, Sanofi Pasteur, and Thermo Fisher. FLJ reports grant from the Coalition of Epidemic Preparedness Innovations (CEPI), grant from South-East region Health authority. KKJ reports speakers bureaus from Roche and BMS, advisory board Celltrion and Norgine. GLG reports funding from The Karin Fossum foundation, Diakonhjemmet Hospital, Oslo University Hospital, Akershus University Hospital, Trygve Gydtfeldt og frues Foundation, South-East region Health authority, consulting fees AbbVie and Pfizer, speaker’s fees AbbVie, Pfizer, Sandoz, Orion Pharma, Novartis and UCB, advisory board Pfizer, AbbVie. IEC, IJ, SWS, ATT, TTT, JS, SAP, SM, DJW, GBK, and EAH report nothing to disclose.
Figures

Similar articles
-
Immunogenicity and safety of a three-dose SARS-CoV-2 vaccination strategy in patients with immune-mediated inflammatory diseases on immunosuppressive therapy.RMD Open. 2022 Nov;8(2):e002417. doi: 10.1136/rmdopen-2022-002417. RMD Open. 2022. PMID: 36328399 Free PMC article.
-
Immunogenicity and Safety of Standard and Third-Dose SARS-CoV-2 Vaccination in Patients Receiving Immunosuppressive Therapy.Arthritis Rheumatol. 2022 Aug;74(8):1321-1332. doi: 10.1002/art.42153. Epub 2022 Jun 29. Arthritis Rheumatol. 2022. PMID: 35507355 Free PMC article.
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Four SARS-CoV-2 vaccine doses or hybrid immunity in patients on immunosuppressive therapies: a Norwegian cohort study.Lancet Rheumatol. 2023 Jan;5(1):e36-e46. doi: 10.1016/S2665-9913(22)00330-7. Epub 2022 Nov 16. Lancet Rheumatol. 2023. PMID: 36415604 Free PMC article.
-
Immunosuppression in Glomerular Diseases: Implications for SARS-CoV-2 Vaccines and COVID-19.Glomerular Dis. 2021 Aug 25;1(4):277-293. doi: 10.1159/000519182. eCollection 2021 Oct. Glomerular Dis. 2021. PMID: 34935004 Free PMC article. Review.
Cited by
-
Humoral and cellular responses to a fifth bivalent SARS-CoV-2 vaccine dose in patients with immune-mediated inflammatory diseases on tumour necrosis factor inhibitors: a prospective cohort study.Lancet Reg Health Eur. 2024 Nov 15;48:101121. doi: 10.1016/j.lanepe.2024.101121. eCollection 2025 Jan. Lancet Reg Health Eur. 2024. PMID: 39624496 Free PMC article.
-
Duration of Postvaccination Neutralizing Antibodies to SARS-CoV-2 and Medication Effects: Results from the Safety and Immunogenicity of COVID-19 Vaccination in Systemic Immune-Mediated Inflammatory Diseases Cohort Study.ACR Open Rheumatol. 2024 Sep;6(9):581-586. doi: 10.1002/acr2.11697. Epub 2024 Jul 1. ACR Open Rheumatol. 2024. PMID: 38952080 Free PMC article.
-
Immunogenicity and safety of a three-dose SARS-CoV-2 vaccination strategy in patients with immune-mediated inflammatory diseases on immunosuppressive therapy.RMD Open. 2022 Nov;8(2):e002417. doi: 10.1136/rmdopen-2022-002417. RMD Open. 2022. PMID: 36328399 Free PMC article.
-
Adaptive immune responses to SARS-CoV-2 in DMARD-treated patients with chronic inflammatory rheumatisms.RMD Open. 2025 Jul 5;11(3):e005673. doi: 10.1136/rmdopen-2025-005673. RMD Open. 2025. PMID: 40617588 Free PMC article.
-
Did We Overreact? Insights on COVID-19 Disease and Vaccination in a Large Cohort of Immune-Mediated Inflammatory Disease Patients during Sequential Phases of the Pandemic (The BELCOMID Study).Vaccines (Basel). 2024 Oct 11;12(10):1157. doi: 10.3390/vaccines12101157. Vaccines (Basel). 2024. PMID: 39460324 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

