Greater Plasma Protein Adsorption on Mesoporous Silica Nanoparticles Aggravates Atopic Dermatitis
- PMID: 36199478
- PMCID: PMC9528962
- DOI: 10.2147/IJN.S383324
Greater Plasma Protein Adsorption on Mesoporous Silica Nanoparticles Aggravates Atopic Dermatitis
Abstract
Purpose: The protein corona surrounding nanoparticles has attracted considerable attention as it induces subsequent inflammatory responses. Although mesoporous silica nanoparticles (MSN) are commonly used in medicines, cosmetics, and packaging, the inflammatory effects of the MSN protein corona on the cutaneous system have not been investigated till date.
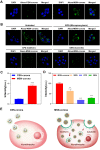
Methods: We examined the greater plasma protein adsorption on MSN leads to serious inflammatory reactions in Dermatophagoides farinae extract (DFE)-induced mouse atopic dermatitis (AD)-like skin inflammation because of increased uptake by keratinocytes.
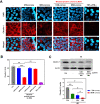
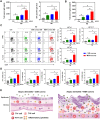
Results: We compare the AD lesions induced by MSN and colloidal (non-porous) silica nanoparticles (CSN), which exhibit different pore architectures but similar dimensions and surface chemistry. MSN-corona treatment of severe skin inflammation in a DFE-induced in vivo AD model greatly increases mouse ear epidermal thickness and infiltration of immune cells compared with the CSN-corona treatment. Moreover, MSN-corona significantly increase AD-specific immunoglobulins, serum histamine, and Th1/Th2/Th17 cytokines in the ear and lymph nodes. MSN-corona induce more severe cutaneous inflammation than CSN by significantly decreasing claudin-1 expression.
Conclusion: This study demonstrates the novel impact of the MSN protein corona in inducing inflammatory responses through claudin-1 downregulation and suggests useful clinical guidelines for MSN application in cosmetics and drug delivery systems.
Keywords: atopic dermatitis; claudin-1; colloidal silica; immunotoxicity; mesoporous silica; protein Corona.
© 2022 Choi et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






Similar articles
-
I. inflexus (Thunb.) Kudo extract improves atopic dermatitis and depressive-like behavior in DfE-induced atopic dermatitis-like disease.Phytomedicine. 2020 Feb;67:153137. doi: 10.1016/j.phymed.2019.153137. Epub 2019 Nov 18. Phytomedicine. 2020. PMID: 31918393
-
Topical application of Moringa oleifera leaf extract ameliorates experimentally induced atopic dermatitis by the regulation of Th1/Th2/Th17 balance.Biomed Pharmacother. 2016 Dec;84:870-877. doi: 10.1016/j.biopha.2016.09.085. Epub 2016 Oct 12. Biomed Pharmacother. 2016. PMID: 27744247
-
Gardenia jasminoides extract without crocin improved atopic dermatitis-like skin lesions via suppression of Th2-related cytokines in Dfe-induced NC/Nga mice.J Ethnopharmacol. 2019 Sep 15;241:112015. doi: 10.1016/j.jep.2019.112015. Epub 2019 Jun 4. J Ethnopharmacol. 2019. PMID: 31173875
-
Mesoporous silica nanoparticles in drug delivery and biomedical applications.Nanomedicine. 2015 Feb;11(2):313-27. doi: 10.1016/j.nano.2014.09.014. Epub 2014 Nov 13. Nanomedicine. 2015. PMID: 25461284 Review.
-
Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents.Molecules. 2020 Aug 21;25(17):3814. doi: 10.3390/molecules25173814. Molecules. 2020. PMID: 32825791 Free PMC article. Review.
Cited by
-
Silica-based microencapsulation used in topical dermatologic applications.Arch Dermatol Res. 2023 Dec;315(10):2787-2793. doi: 10.1007/s00403-023-02725-z. Epub 2023 Oct 4. Arch Dermatol Res. 2023. PMID: 37792034 Free PMC article. Review.
-
Novel Vehicles For Drug Delivery in Atopic Dermatitis: A Narrative Review.Dermatol Pract Concept. 2023 Oct 1;13(4):e2023216. doi: 10.5826/dpc.1304a216. Dermatol Pract Concept. 2023. PMID: 37992345 Free PMC article. Review.
-
Molybdenum Nanoparticles Alleviate MC903-Induced Atopic Dermatitis-Like Symptoms in Mice by Modulating the ROS-Mediated NF-κB and Nrf2 /HO-1 Signaling Pathways.Int J Nanomedicine. 2024 Aug 27;19:8779-8796. doi: 10.2147/IJN.S472999. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39220192 Free PMC article.
-
Mesoporous Silica-Based Nanoplatforms Are Theranostic Agents for the Treatment of Inflammatory Disorders.Pharmaceutics. 2023 Jan 28;15(2):439. doi: 10.3390/pharmaceutics15020439. Pharmaceutics. 2023. PMID: 36839761 Free PMC article. Review.
-
Combined Therapy of Experimental Autoimmune Uveitis by a Dual-Drug Nanocomposite Formulation with Berberine and Dexamethasone.Int J Nanomedicine. 2023 Jul 31;18:4347-4363. doi: 10.2147/IJN.S417750. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37545873 Free PMC article.
References
-
- Athinarayanan J, Periasamy VS, Alsaif MA, Al-Warthan AA, Alshatwi AA. Presence of nanosilica (E551) in commercial food products: TNF-mediated oxidative stress and altered cell cycle progression in human lung fibroblast cells. Cell Biol Toxicol. 2014;30(2):89–100. doi:10.1007/s10565-014-9271-8 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

