Guanine inhibits the growth of human glioma and melanoma cell lines by interacting with GPR23
- PMID: 36199684
- PMCID: PMC9527276
- DOI: 10.3389/fphar.2022.970891
Guanine inhibits the growth of human glioma and melanoma cell lines by interacting with GPR23
Abstract
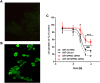
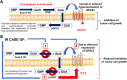
Guanine-based purines (GBPs) exert numerous biological effects at the central nervous system through putative membrane receptors, the existence of which is still elusive. To shed light on this question, we screened orphan and poorly characterized G protein-coupled receptors (GPRs), selecting those that showed a high purinoreceptor similarity and were expressed in glioma cells, where GBPs exerted a powerful antiproliferative effect. Of the GPRs chosen, only the silencing of GPR23, also known as lysophosphatidic acid (LPA) 4 receptor, counteracted GBP-induced growth inhibition in U87 cells. Guanine (GUA) was the most potent compound behind the GPR23-mediated effect, acting as the endpoint effector of GBP antiproliferative effects. Accordingly, cells stably expressing GPR23 showed increased sensitivity to GUA. Furthermore, while GPR23 expression was low in a hypoxanthine-guanine phosphoribosyl-transferase (HGPRT)-mutated melanoma cell line showing poor sensitivity to GBPs, and in HGPRT-silenced glioma cells, GPR23-induced expression in both cell types rescued GUA-mediated cell growth inhibition. Finally, binding experiments using [3H]-GUA and U87 cell membranes revealed the existence of a selective GUA binding (KD = 29.44 ± 4.07 nM; Bmax 1.007 ± 0.035 pmol/mg prot) likely to GPR23. Overall, these data suggest GPR23 involvement in modulating responses to GUA in tumor cell lines, although further research needs to verify whether this receptor mediates other GUA effects.
Keywords: G protein-coupled receptor 23 (GPR23); antiproliferative effects; glioma cell lines; guanine (GUA); guanine-based purines (GBPs); lysophosphatidic acid (LPA); melanoma cell lines; purine nucleoside phosphorylase (PNP).
Copyright © 2022 Garozzo, Zuccarini, Giuliani, Di Liberto, Mudò, Caciagli, Ciccarelli, Ciruela, Di Iorio and Condorelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Abbracchio M. P., Boeynaems J. M., Barnard E. A., Boyer J. L., Kennedy C., Portugal M. T., et al. (2003). Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol. Sci. 24, 52–55. 10.1016/S0165-6147(02)00038-X - DOI - PMC - PubMed
-
- Bender E., Buist A., Jurzak M., Langlois X., Baggerman G., Verhasselt P., et al. (2002). Characterization of an orphan G protein-coupled receptor localized in the dorsal root ganglia reveals adenine as a signaling molecule. Proc. Natl. Acad. Sci. U. S. A. 99, 8573–8578. 10.1073/pnas.122016499 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

