Novel Drug Design for Treatment of COVID-19: A Systematic Review of Preclinical Studies
- PMID: 36199815
- PMCID: PMC9527439
- DOI: 10.1155/2022/2044282
Novel Drug Design for Treatment of COVID-19: A Systematic Review of Preclinical Studies
Abstract
Background: Since the beginning of the novel coronavirus (SARS-CoV-2) disease outbreak, there has been an increasing interest in discovering potential therapeutic agents for this disease. In this regard, we conducted a systematic review through an overview of drug development (in silico, in vitro, and in vivo) for treating COVID-19.
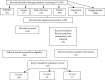
Methods: A systematic search was carried out in major databases including PubMed, Web of Science, Scopus, EMBASE, and Google Scholar from December 2019 to March 2021. A combination of the following terms was used: coronavirus, COVID-19, SARS-CoV-2, drug design, drug development, In silico, In vitro, and In vivo. A narrative synthesis was performed as a qualitative method for the data synthesis of each outcome measure.
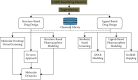
Results: A total of 2168 articles were identified through searching databases. Finally, 315 studies (266 in silico, 34 in vitro, and 15 in vivo) were included. In studies with in silico approach, 98 article study repurposed drug and 91 studies evaluated herbal medicine on COVID-19. Among 260 drugs repurposed by the computational method, the best results were observed with saquinavir (n = 9), ritonavir (n = 8), and lopinavir (n = 6). Main protease (n = 154) following spike glycoprotein (n = 62) and other nonstructural protein of virus (n = 45) was among the most studied targets. Doxycycline, chlorpromazine, azithromycin, heparin, bepridil, and glycyrrhizic acid showed both in silico and in vitro inhibitory effects against SARS-CoV-2.
Conclusion: The preclinical studies of novel drug design for COVID-19 focused on main protease and spike glycoprotein as targets for antiviral development. From evaluated structures, saquinavir, ritonavir, eucalyptus, Tinospora cordifolia, aloe, green tea, curcumin, pyrazole, and triazole derivatives in in silico studies and doxycycline, chlorpromazine, and heparin from in vitro and human monoclonal antibodies from in vivo studies showed promised results regarding efficacy. It seems that due to the nature of COVID-19 disease, finding some drugs with multitarget antiviral actions and anti-inflammatory potential is valuable and some herbal medicines have this potential.
Copyright © 2022 Sarah Mousavi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Targeted therapy strategies against SARS-CoV-2 cell entry mechanisms: A systematic review of in vitro and in vivo studies.J Cell Physiol. 2021 Apr;236(4):2364-2392. doi: 10.1002/jcp.30032. Epub 2020 Sep 9. J Cell Physiol. 2021. PMID: 32901936
-
Mechanistic Aspects of Medicinal Plants and Secondary Metabolites against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).Curr Pharm Des. 2021;27(38):3996-4007. doi: 10.2174/1381612827666210705160130. Curr Pharm Des. 2021. PMID: 34225607 Review.
-
Computational drug discovery and repurposing for the treatment of COVID-19: A systematic review.Bioorg Chem. 2021 Jan;106:104490. doi: 10.1016/j.bioorg.2020.104490. Epub 2020 Nov 19. Bioorg Chem. 2021. PMID: 33261845 Free PMC article.
-
Molecular Docking of Azithromycin, Ritonavir, Lopinavir, Oseltamivir, Ivermectin and Heparin Interacting with Coronavirus Disease 2019 Main and Severe Acute Respiratory Syndrome Coronavirus-2 3C-Like Proteases.J Nanosci Nanotechnol. 2021 Apr 1;21(4):2075-2089. doi: 10.1166/jnn.2021.19029. J Nanosci Nanotechnol. 2021. PMID: 33500022
-
Computational drug repurposing study of antiviral drugs against main protease, RNA polymerase, and spike proteins of SARS-CoV-2 using molecular docking method.J Basic Clin Physiol Pharmacol. 2021 Jul 15;33(1):85-95. doi: 10.1515/jbcpp-2020-0369. J Basic Clin Physiol Pharmacol. 2021. PMID: 34265888
Cited by
-
Special Issue: "Rational Design and Synthesis of Bioactive Molecules".Int J Mol Sci. 2024 Sep 14;25(18):9927. doi: 10.3390/ijms25189927. Int J Mol Sci. 2024. PMID: 39337415 Free PMC article.
-
Repositioned Natural Compounds and Nanoformulations: A Promising Combination to Counteract Cell Damage and Inflammation in Respiratory Viral Infections.Molecules. 2023 May 12;28(10):4045. doi: 10.3390/molecules28104045. Molecules. 2023. PMID: 37241786 Free PMC article. Review.
-
Discovering broad-spectrum inhibitors for SARS-CoV-2 variants: a cheminformatics and biophysical approach targeting the main protease.Front Pharmacol. 2025 Feb 5;16:1459581. doi: 10.3389/fphar.2025.1459581. eCollection 2025. Front Pharmacol. 2025. PMID: 39974733 Free PMC article.
-
De Novo Potent Peptide Nucleic Acid Antisense Oligomer Inhibitors Targeting SARS-CoV-2 RNA-Dependent RNA Polymerase via Structure-Guided Drug Design.Int J Mol Sci. 2023 Dec 14;24(24):17473. doi: 10.3390/ijms242417473. Int J Mol Sci. 2023. PMID: 38139312 Free PMC article.
-
Paving New Roads Using Allium sativum as a Repurposed Drug and Analyzing its Antiviral Action Using Artificial Intelligence Technology.Iran J Pharm Res. 2023 Jan 21;21(1):e131577. doi: 10.5812/ijpr-131577. eCollection 2022 Dec. Iran J Pharm Res. 2023. PMID: 36915406 Free PMC article. Review.
References
-
- WHO. WHO coronavirus (COVID-19) dashboard. 2022. https://covid19.who.int/
-
- WHO. Therapeutics and COVID-19: Living Guideline . Geneva, Swizterland: World Health Organization; 2022. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous