Escitalopram co-prescription in anastrozole-treated breast cancer patients
- PMID: 36199859
- PMCID: PMC9464843
- DOI: 10.14744/nci.2022.48264
Escitalopram co-prescription in anastrozole-treated breast cancer patients
Abstract
Objective: The purpose of the study was to evaluate the impact of escitalopram co-prescription on plasma anastrozole levels in post-menopausal breast cancer patients.
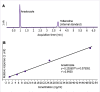
Methods: A total of 24 post-menopausal operated breast cancer patients co-prescribed with escitalopram and anastrozole were included. Blood samples were collected, before and 1-month after the onset of escitalopram to analyze plasma anastrozole and estradiol levels.
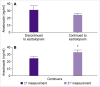
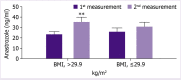
Results: No significant difference was noted in basal plasma anastrozole levels with respect to age, body mass index (BMI), tumor stage, previous antineoplastic treatments, concomitant medications, and serum estradiol levels. Overall, 17 patients completed the 1-month escitalopram treatment, while 7 patients discontinued escitalopram within the 1st week of the treatment. Basal anastrozole levels of 24 patients were 26.1±2.4 ng/mL. Among 17 patients who continued 1-month escitalopram treatment was associated with significant increase in plasma anastrozole levels (24.5±2.3 ng/mL to 32.2±3.2 ng/mL, p<0.05). Notably, 1-month escitalopram use was associated with significant increase in plasma anastrozole levels only in the subgroup of obese (BMI >29 kg/m2) patients (23.1±2.8 to 35.9±4.7 ng/mL, p<0.01), while no such interaction was noted among non-obese patients. The estradiol levels of the patients were below ≤10 pg/mL in 75% of patients and no change occurred after escitalopram administration.
Conclusion: Escitalopram co-prescription resulted in significant increase in plasma anastrozole levels without affecting the serum estradiol levels. Our findings emphasize the need for close monitoring in case of concomitant use of anastrozole and escitalopram, especially in obese patients and the potential role of therapeutic drug monitoring.
Keywords: Anastrozole; breast cancer; escitalopram; obesity; therapeutic drug monitoring.
© Copyright 2022 by Istanbul Provincial Directorate of Health.
Conflict of interest statement
No conflict of interest was declared by the authors.
Figures
Similar articles
-
Does obesity interfere with anastrozole treatment? Positive association between body mass index and anastrozole plasma levels.Clin Breast Cancer. 2014 Aug;14(4):291-6. doi: 10.1016/j.clbc.2013.12.008. Epub 2013 Dec 27. Clin Breast Cancer. 2014. PMID: 24468298
-
Can we use gonadotropin plasma concentration as surrogate marker for BMI-related incomplete estrogen suppression in breast cancer patients receiving anastrozole?BMC Cancer. 2017 Mar 28;17(1):226. doi: 10.1186/s12885-017-3208-6. BMC Cancer. 2017. PMID: 28351392 Free PMC article.
-
Estrone and Estradiol Levels in Breast Cancer Patients Using Anastrozole Are Not Related to Body Mass Index.Rev Bras Ginecol Obstet. 2017 Jan;39(1):14-20. doi: 10.1055/s-0036-1597974. Epub 2017 Feb 10. Rev Bras Ginecol Obstet. 2017. PMID: 28187491
-
Impact of body mass index on estradiol depletion by aromatase inhibitors in postmenopausal women with early breast cancer.Br J Cancer. 2013 Sep 17;109(6):1522-7. doi: 10.1038/bjc.2013.499. Epub 2013 Sep 3. Br J Cancer. 2013. PMID: 24002592 Free PMC article.
-
ARIMIDEX: a potent and selective aromatase inhibitor for the treatment of advanced breast cancer.J Steroid Biochem Mol Biol. 1997 Apr;61(3-6):145-9. J Steroid Biochem Mol Biol. 1997. PMID: 9365184 Review.
References
-
- Abubakar MB, Wei K, Gan SH. The influence of genetic polymorphisms on the efficacy and side effects of anastrozole in postmenopausal breast cancer patients. Pharmacogenet Genomics. 2014;24:575–81. - PubMed
-
- Ponzone R, Mininanni P, Cassina E, Pastorino F, Sismondi P. Aromatase inhibitors for breast cancer: different structures, same effects? Endocr Relat Cancer. 2008;15:27–36. - PubMed
-
- Ingle JN, Dowsett M, Cuzick J, Davies C. Aromatase inhibitors versus tamoxifen as adjuvant therapy for postmenopausal women with estrogen receptor positive breast cancer: meta-analyses of randomized trials of monotherapy and switching strategies. Cancer Res. 2009;69:66.
-
- Arimidex Tamoxifen Alone or in Combination (ATAC) Trialists’ Group. Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. - PubMed
LinkOut - more resources
Full Text Sources
