Chemical diversity, medicinal potentialities, biosynthesis, and pharmacokinetics of anthraquinones and their congeners derived from marine fungi: a comprehensive update
- PMID: 36199881
- PMCID: PMC9434105
- DOI: 10.1039/d2ra03610j
Chemical diversity, medicinal potentialities, biosynthesis, and pharmacokinetics of anthraquinones and their congeners derived from marine fungi: a comprehensive update
Abstract
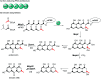
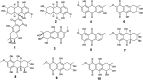
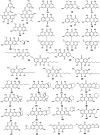
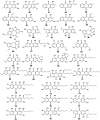
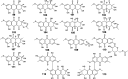
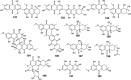
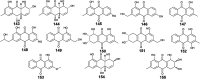
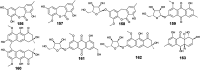
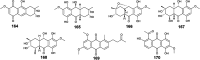
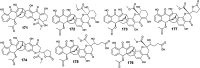
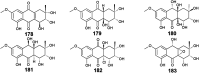
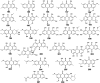
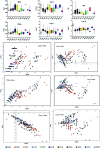
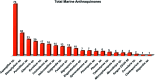
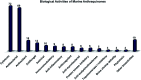
Marine fungi receive excessive attention as prolific producers of structurally unique secondary metabolites, offering promising potential as substitutes or conjugates for current therapeutics, whereas existing research has only scratched the surface in terms of secondary metabolite diversity and potential industrial applications as only a small share of bioactive natural products have been identified from marine-derived fungi thus far. Anthraquinones derived from filamentous fungi are a distinct large group of polyketides containing compounds which feature a common 9,10-dioxoanthracene core, while their derivatives are generated through enzymatic reactions such as methylation, oxidation, or dimerization to produce a large variety of anthraquinone derivatives. A considerable number of reported anthraquinones and their derivatives have shown significant biological activities as well as highly economical, commercial, and biomedical potentialities such as anticancer, antimicrobial, antioxidant, and anti-inflammatory activities. Accordingly, and in this context, this review comprehensively covers the state-of-art over 20 years of about 208 structurally diverse anthraquinones and their derivatives isolated from different species of marine-derived fungal genera along with their reported bioactivity wherever applicable. Also, in this manuscript, we will present in brief recent insights centred on their experimentally proved biosynthetic routes. Moreover, all reported compounds were extensively investigated for their in-silico drug-likeness and pharmacokinetics properties which intriguingly highlighted a list of 20 anthraquinone-containing compounds that could be considered as potential drug lead scaffolds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing commercial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Newman D. J. Cragg G. M. J. Nat. Prod. 2016;79:629–661. - PubMed
-
- Ghareeb M. A. Tammam M. A. El-Demerdash A. Atanasov A. G. Curr. Res. Biotechnol. 2020;2:88–102.
Publication types
LinkOut - more resources
Full Text Sources

