Recent progress of Ga-based liquid metals in catalysis
- PMID: 36199892
- PMCID: PMC9434383
- DOI: 10.1039/d2ra04795k
Recent progress of Ga-based liquid metals in catalysis
Abstract
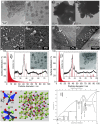
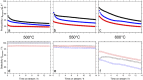
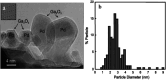
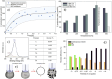
Within the last decade, the application of gallium-based liquid metals in catalysis has received great attention from around the world. This article provides an overview concerning Ga-based liquid metals (LMs) in energy and environmental applications, such as the catalytic synthesis of ethylene by non-petroleum routes via Pd-Ga liquid catalysts, alkane dehydrogenation via Pd-Ga or Pt-Ga catalysts, CO2 hydrogenation to methanol via Ni Ga or Pd/Ga2O3 catalysts, and catalytic degradation of CO2 via EGaIn liquid metal catalysts below 500 °C, where Ga-based liquid metal catalysts exhibit high selectivity and low energy consumption. The formation of isolated metal sites in a liquid metal matrix allows the integration of several characteristics of multiphase catalysis (particularly the operational friendliness of product separation procedures) with those of homogeneous catalysis. In the end, this article sheds light on future prospects, opportunities, and challenges of liquid metal catalysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Wang Q. Yu Y. Liu J. Adv. Eng. Mater. 2018;20:1700781.
-
- Dickey M. D. Chiechi R. C. Larsen R. J. Weiss E. A. Weitz D. A. Whitesides G. M. Adv. Funct. Mater. 2008;18:1097–1104.
-
- Calabrese R. E. Bury E. Haque F. Koh A. Park C. Composites, Part B. 2022;234:109686.
-
- Ozutemiz K. B. Wissman J. Ozdoganlar O. B. Majidi C. Adv. Mater. Interfaces. 2018;5:1701596.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

