Comparison of continuous glucose monitoring to reference standard oral glucose tolerance test for the detection of dysglycemia in cystic Fibrosis: A systematic review
- PMID: 36200022
- PMCID: PMC9529501
- DOI: 10.1016/j.jcte.2022.100305
Comparison of continuous glucose monitoring to reference standard oral glucose tolerance test for the detection of dysglycemia in cystic Fibrosis: A systematic review
Abstract
Aims: Increasing evidence for benefit of early detection of cystic fibrosis related diabetes (CFRD) coupled with limitations of current diagnostic investigations has led to interest and utilisation of continuous glucose monitoring (CGM). We conducted a systematic review to assess current evidence on CGM compared to reference standard oral glucose tolerance test for the detection of dysglycemia in people with cystic fibrosis without confirmed diabetes.
Methods: MEDLINE, Embase, CENTRAL, Evidence-Based Medicine Reviews, grey literature and six relevant journals were searched for studies published after year 2000. Studies reporting contemporaneous CGM metrics and oral glucose tolerance test results were included. Outcomes on oral glucose tolerance tests were categorised into a) normal, b) abnormal (indeterminate and impaired) or c) diabetic as defined by American Diabetes Association criteria. CGM outcomes were defined as hyperglycemia (≥1 peak sensor glucose ≥ 200 mg/dL), dysglycemia (≥1 peak sensor glucose ≥ 140-199 mg/dL) or normoglycemia (all sensor glucose peaks < 140 mg/dL). CGM hyperglycemia in people with normal or abnormal glucose tolerances was used to define an arbitrary CGM-diagnosis of diabetes. The Quality Assessment of Diagnostic Accuracy Studies tool was used to assess risk of bias. Primary outcome was relative risk of an arbitrary CGM-diagnosis of diabetes compared to the oral glucose tolerance test.
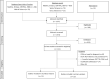
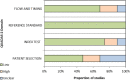
Results: We identified 1277 publications, of which 19 studies were eligible comprising total of 416 individuals with contemporaneous CGM and oral glucose tolerance test results. Relative risk of an arbitrary CGM-diagnosis of diabetes compared to oral glucose tolerance test was 2.92. Studies analysed were highly heterogenous, prone to bias and inadequately assessed longitudinal associations between CGM and relevant disease-specific sequela.
Conclusions: A single reading > 200 mg/dL on CGM is not appropriate for the diagnosis of CFRD. Prospective studies correlating CGM metrics to disease-specific outcomes are needed to determine appropriate cut-points.
Keywords: Continuous glucose monitoring; Cystic fibrosis; Diabetes; Diagnosis.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
The Role of Continuous Glucose Monitoring in Detecting Early Dysglycemia and Clinical Outcomes in Patients with Cystic Fibrosis.Medicina (Kaunas). 2024 Mar 14;60(3):477. doi: 10.3390/medicina60030477. Medicina (Kaunas). 2024. PMID: 38541202 Free PMC article. Review.
-
Continuous glucose monitoring and advanced glycation endproducts for prediction of clinical outcomes and development of cystic fibrosis-related diabetes in adults with CF.Front Endocrinol (Lausanne). 2024 Feb 6;15:1293709. doi: 10.3389/fendo.2024.1293709. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38379863 Free PMC article.
-
Oral glucose tolerance test and continuous glucose monitoring to assess diabetes development in cystic fibrosis patients.Endocrinol Diabetes Nutr (Engl Ed). 2018 Jan;65(1):45-51. doi: 10.1016/j.endinu.2017.08.008. Epub 2017 Nov 12. Endocrinol Diabetes Nutr (Engl Ed). 2018. PMID: 29137964 Clinical Trial. English, Spanish.
-
Continuous Glucose Monitoring and HbA1c in Cystic Fibrosis: Clinical Correlations and Implications for CFRD Diagnosis.J Clin Endocrinol Metab. 2022 Mar 24;107(4):e1444-e1454. doi: 10.1210/clinem/dgab857. J Clin Endocrinol Metab. 2022. PMID: 34850006 Free PMC article.
-
Drug treatments for managing cystic fibrosis-related diabetes.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD004730. doi: 10.1002/14651858.CD004730.pub5. Cochrane Database Syst Rev. 2020. PMID: 33075159 Free PMC article.
Cited by
-
The Role of Continuous Glucose Monitoring in Detecting Early Dysglycemia and Clinical Outcomes in Patients with Cystic Fibrosis.Medicina (Kaunas). 2024 Mar 14;60(3):477. doi: 10.3390/medicina60030477. Medicina (Kaunas). 2024. PMID: 38541202 Free PMC article. Review.
-
Advances in diabetes technology to improve the lives of people with cystic fibrosis.Diabetologia. 2024 Oct;67(10):2143-2153. doi: 10.1007/s00125-024-06223-3. Epub 2024 Jul 12. Diabetologia. 2024. PMID: 38995399 Review.
-
Expanding the Role of Continuous Glucose Monitoring in Modern Diabetes Care Beyond Type 1 Disease.Diabetes Ther. 2023 Aug;14(8):1241-1266. doi: 10.1007/s13300-023-01431-3. Epub 2023 Jun 16. Diabetes Ther. 2023. PMID: 37322319 Free PMC article. Review.
References
-
- Mirtajani S., et al. Geographical distribution of cystic fibrosis; The past 70 years of data analyzis. Biomedical and Biotechnology Research Journal (BBRJ) 2017;1(2):105–112.
-
- Dupuis A., et al. Cystic fibrosis birth rates in canada: a decreasing trend since the onset of genetic testing. J Ped. 2005;147(3):312–315. - PubMed
Publication types
LinkOut - more resources
Full Text Sources