A computational protocol for the calculation of the standard reduction potential of iron complexes: application to Fe2+/3+-Aβ model systems relevant to Alzheimer's disease
- PMID: 36200023
- PMCID: PMC9451132
- DOI: 10.1039/d2ra03907a
A computational protocol for the calculation of the standard reduction potential of iron complexes: application to Fe2+/3+-Aβ model systems relevant to Alzheimer's disease
Abstract
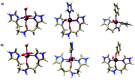
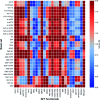
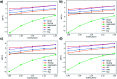
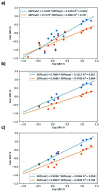
Iron complexes play a key role in several biological processes, and they are also related to the development of neurological disorders, such as Alzheimer's and Parkinson's diseases. One of the main properties involved in these processes is the standard reduction potential (SRP) of iron complexes. However, the calculation of this property is challenging, mainly due to problems in the electronic structure description, solvent effects and the thermodynamic cycles used for its calculation. In this work, we proposed a computational protocol for the calculation of SRPs of iron complexes by evaluating a wide range of density functionals for the electronic structure description, two implicit solvent models with varying radii and two thermodynamic cycles. Results show that the M06L density functional in combination with the SMD solvation model and the isodesmic method provides good results compared with SRP experimental values for a set of iron complexes. Finally, this protocol was applied to three Fe2+/3+-Aβ model systems involved in the development of Alzheimer's disease and the obtained SRP values are in good agreement with those reported previously by means of MP2 calculations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



Similar articles
-
Assessment of the isodesmic method in the calculation of standard reduction potential of copper complexes.J Mol Model. 2017 Sep 21;23(10):283. doi: 10.1007/s00894-017-3469-7. J Mol Model. 2017. PMID: 28936691
-
Computational Design of Copper Ligands with Controlled Metal Chelating, Pharmacokinetics, and Redox Properties for Alzheimer's Disease.J Alzheimers Dis. 2021;82(s1):S179-S193. doi: 10.3233/JAD-200911. J Alzheimers Dis. 2021. PMID: 34032611
-
Benchmark of Density Functionals for the Calculation of the Redox Potential of Fe3+/Fe2+ Within Protein Coordination Shells.Front Chem. 2019 Jun 5;7:391. doi: 10.3389/fchem.2019.00391. eCollection 2019. Front Chem. 2019. PMID: 31231631 Free PMC article.
-
Distinct role of hydration water in protein misfolding and aggregation revealed by fluctuating thermodynamics analysis.Acc Chem Res. 2015 Apr 21;48(4):956-65. doi: 10.1021/acs.accounts.5b00032. Epub 2015 Apr 6. Acc Chem Res. 2015. PMID: 25844814 Review.
-
Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics.J Neurochem. 2016 Oct;139 Suppl 1:179-197. doi: 10.1111/jnc.13425. Epub 2016 Feb 10. J Neurochem. 2016. PMID: 26545340 Review.
Cited by
-
Role of Metal Cations of Copper, Iron, and Aluminum and Multifunctional Ligands in Alzheimer's Disease: Experimental and Computational Insights.ACS Omega. 2023 Jan 25;8(5):4508-4526. doi: 10.1021/acsomega.2c06939. eCollection 2023 Feb 7. ACS Omega. 2023. PMID: 36777601 Free PMC article. Review.
References
-
- Belaidi A. A. Bush A. I. Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. J. Neurochem. 2016:179–197. - PubMed
-
- Mochizuki H. Choong C. J. Baba K. Parkinson's disease and iron. J. Neural Transm. 2020;127:181–187. - PubMed
-
- Mostile G. Cicero C. E. Giuliano L. Zappia M. Nicoletti A. Iron and Parkinson's disease: a systematic review and meta-analysis. Mol. Med. Rep. 2017;15:3383–3389. - PubMed
-
- Rauk A. The chemistry of Alzheimer's disease. Chem. Soc. Rev. 2009;38:2698. - PubMed
LinkOut - more resources
Full Text Sources

