Microtubule motor driven interactions of lipid droplets: Specificities and opportunities
- PMID: 36200039
- PMCID: PMC9527339
- DOI: 10.3389/fcell.2022.893375
Microtubule motor driven interactions of lipid droplets: Specificities and opportunities
Abstract
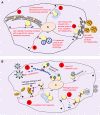
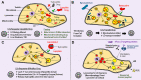
Lipid Droplets (LDs) are evolutionarily conserved cellular organelles that store neutral lipids such as triacylglycerol and cholesterol-esters. Neutral lipids are enclosed within the limiting membrane of the LD, which is a monolayer of phospholipids and is therefore fundamentally different from the bilayer membrane enclosing most other organelles. LDs have long been viewed as a storehouse of lipids needed on demand for generating energy and membranes inside cells. Outside this classical view, we are now realizing that LDs have significant roles in protein sequestration, supply of signalling lipids, viral replication, lipoprotein production and many other functions of important physiological consequence. To execute such functions, LDs must often exchange lipids and proteins with other organelles (e.g., the ER, lysosomes, mitochondria) via physical contacts. But before such exchanges can occur, how does a micron-sized LD with limited ability to diffuse around find its cognate organelle? There is growing evidence that motor protein driven motion of LDs along microtubules may facilitate such LD-organelle interactions. We will summarize some aspects of LD motion leading to LD-organelle contacts, how these change with metabolic state and pathogen infections, and also ask how these pathways could perhaps be targeted selectively in the context of disease and drug delivery. Such a possibility arises because the binding of motor proteins to the monolayer membrane on LDs could be different from motor binding to the membrane on other cellular organelles.
Keywords: drug delivery; dynein; kinesin; lipid droplet; lipid metabolism; membrane contacts; microtubule motor; pathogen.
Copyright © 2022 Singh, Sanghavi and Mallik.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Lipid droplet motility and organelle contacts.Contact (Thousand Oaks). 2019 Jan-Dec;2:10.1177/2515256419895688. doi: 10.1177/2515256419895688. Epub 2019 Dec 16. Contact (Thousand Oaks). 2019. PMID: 31909374 Free PMC article.
-
Membrane shaping proteins, lipids, and cytoskeleton: Recipe for nascent lipid droplet formation.Bioessays. 2022 Sep;44(9):e2200038. doi: 10.1002/bies.202200038. Epub 2022 Jul 13. Bioessays. 2022. PMID: 35832014
-
ORP5 localizes to ER-lipid droplet contacts and regulates the level of PI(4)P on lipid droplets.J Cell Biol. 2020 Jan 6;219(1):e201905162. doi: 10.1083/jcb.201905162. J Cell Biol. 2020. PMID: 31653673 Free PMC article.
-
A Unique Junctional Interface at Contact Sites Between the Endoplasmic Reticulum and Lipid Droplets.Front Cell Dev Biol. 2021 Apr 8;9:650186. doi: 10.3389/fcell.2021.650186. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33898445 Free PMC article. Review.
-
Friend or Foe: Lipid Droplets as Organelles for Protein and Lipid Storage in Cellular Stress Response, Aging and Disease.Molecules. 2020 Oct 30;25(21):5053. doi: 10.3390/molecules25215053. Molecules. 2020. PMID: 33143278 Free PMC article. Review.
Cited by
-
Tau is required for glial lipid droplet formation and resistance to neuronal oxidative stress.Nat Neurosci. 2024 Oct;27(10):1918-1933. doi: 10.1038/s41593-024-01740-1. Epub 2024 Aug 26. Nat Neurosci. 2024. PMID: 39187706 Free PMC article.
-
Hypolipidemic Effects of Beetroot Juice in SHR-CRP and HHTg Rat Models of Metabolic Syndrome: Analysis of Hepatic Proteome.Metabolites. 2023 Jan 28;13(2):192. doi: 10.3390/metabo13020192. Metabolites. 2023. PMID: 36837811 Free PMC article.
References
-
- Benador I. Y., Veliova M., Mahdaviani K., Petcherski A., Wikstrom J. D., Assali E. A., et al. (2018). Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 27, 869–885. e6. 10.1016/J.CMET.2018.03.003 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

