An in vitro assay for enzymatic studies on human ALG13/14 heterodimeric UDP- N-acetylglucosamine transferase
- PMID: 36200043
- PMCID: PMC9527342
- DOI: 10.3389/fcell.2022.1008078
An in vitro assay for enzymatic studies on human ALG13/14 heterodimeric UDP- N-acetylglucosamine transferase
Abstract
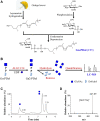
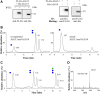
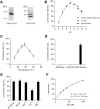
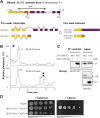
The second step of eukaryotic lipid-linked oligosaccharide (LLO) biosynthesis is catalyzed by the conserved ALG13/ALG14 heterodimeric UDP-N-acetylglucosamine transferase (GnTase). In humans, mutations in ALG13 or ALG14 lead to severe neurological disorders with a multisystem phenotype, known as ALG13/14-CDG (congenital disorders of glycosylation). How these mutations relate to disease is unknown because to date, a reliable GnTase assay for studying the ALG13/14 complex is lacking. Here we describe the development of a liquid chromatography/mass spectrometry-based quantitative GnTase assay using chemically synthesized GlcNAc-pyrophosphate-dolichol as the acceptor and purified human ALG13/14 dimeric enzyme. This assay enabled us to demonstrate that in contrast to the literature, only the shorter human ALG13 isoform 2, but not the longer isoform 1 forms a functional complex with ALG14 that participates in LLO synthesis. The longer ALG13 isoform 1 does not form a complex with ALG14 and therefore lacks GnTase activity. Importantly, we further established a quantitative assay for GnTase activities of ALG13- and ALG14-CDG variant alleles, demonstrating that GnTase deficiency is the cause of ALG13/14-CDG phenotypes.
Keywords: ALG glycosyltransferases; ALG13 isoforms; ALG13/14 UDP-N-acetylglucosamine transferase; N-glycosylation; congenital disorders of glycosylation (CDG); lipid-linked oligosaccharide (LLO).
Copyright © 2022 Wang, Xu, Chen, Chen, Dean, Wang and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Alg14 recruits Alg13 to the cytoplasmic face of the endoplasmic reticulum to form a novel bipartite UDP-N-acetylglucosamine transferase required for the second step of N-linked glycosylation.J Biol Chem. 2005 Oct 28;280(43):36254-62. doi: 10.1074/jbc.M507569200. Epub 2005 Aug 12. J Biol Chem. 2005. PMID: 16100110
-
Interaction between the C termini of Alg13 and Alg14 mediates formation of the active UDP-N-acetylglucosamine transferase complex.J Biol Chem. 2008 Nov 21;283(47):32534-41. doi: 10.1074/jbc.M804060200. Epub 2008 Sep 22. J Biol Chem. 2008. PMID: 18809682 Free PMC article.
-
Alg14 organizes the formation of a multiglycosyltransferase complex involved in initiation of lipid-linked oligosaccharide biosynthesis.Glycobiology. 2012 Apr;22(4):504-16. doi: 10.1093/glycob/cwr162. Epub 2011 Nov 7. Glycobiology. 2012. PMID: 22061998
-
ALG13-Related Epilepsy: Current Insights and Future Research Directions.Neurochem Res. 2024 Dec 14;50(1):60. doi: 10.1007/s11064-024-04300-y. Neurochem Res. 2024. PMID: 39673593 Review.
-
Exome sequence identified a c.320A > G ALG13 variant in a female with infantile epileptic encephalopathy with normal glycosylation and random X inactivation: Review of the literature.Eur J Med Genet. 2017 Oct;60(10):541-547. doi: 10.1016/j.ejmg.2017.07.014. Epub 2017 Aug 1. Eur J Med Genet. 2017. PMID: 28778787 Review.
Cited by
-
ALG13-Congenital Disorder of Glycosylation (ALG13-CDG): Updated clinical and molecular review and clinical management guidelines.Mol Genet Metab. 2024 Jun;142(2):108472. doi: 10.1016/j.ymgme.2024.108472. Epub 2024 Apr 23. Mol Genet Metab. 2024. PMID: 38703411 Free PMC article. Review.
-
Mutations in Glycosyltransferases and Glycosidases: Implications for Associated Diseases.Biomolecules. 2024 Apr 19;14(4):497. doi: 10.3390/biom14040497. Biomolecules. 2024. PMID: 38672513 Free PMC article.
-
Rft1 catalyzes lipid-linked oligosaccharide translocation across the ER membrane.Nat Commun. 2024 Jun 17;15(1):5157. doi: 10.1038/s41467-024-48999-3. Nat Commun. 2024. PMID: 38886340 Free PMC article.
References
-
- Chantret I., Dancourt J., Barbat A., Moore S. E. (2005). Two proteins homologous to the N- and C-terminal domains of the bacterial glycosyltransferase Murg are required for the second step of dolichyl-linked oligosaccharide synthesis in Saccharomyces cerevisiae . J. Biol. Chem. 280, 9236–9242. 10.1074/jbc.M413941200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

