Targeting Gut Dysbiosis and Microbiome Metabolites for the Development of Therapeutic Modalities for Neurological Disorders
- PMID: 36200211
- PMCID: PMC10716879
- DOI: 10.2174/1570159X20666221003085508
Targeting Gut Dysbiosis and Microbiome Metabolites for the Development of Therapeutic Modalities for Neurological Disorders
Abstract
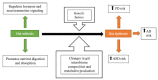
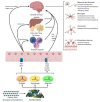
The gut microbiota, composed of numerous species of microbes, works in synergy with the various organ systems in the body to bolster our overall health and well-being. The most well-known function of the gut microbiome is to facilitate the metabolism and absorption of crucial nutrients, such as complex carbohydrates, while also generating vitamins. In addition, the gut microbiome plays a crucial role in regulating the functioning of the central nervous system (CNS). Host genetics, including specific genes and single nucleotide polymorphisms (SNPs), have been implicated in the pathophysiology of neurological disorders, including Parkinson's disease (PD), Alzheimer's disease (AD), and autism spectrum disorder (ASD). The gut microbiome dysbiosis also plays a role in the pathogenesis of these neurodegenerative disorders, thus perturbing the gut-brain axis. Overproduction of certain metabolites synthesized by the gut microbiome, such as short-chain fatty acids (SCFAs) and p-cresyl sulfate, are known to interfere with microglial function and trigger misfolding of alpha-synuclein protein, which can build up inside neurons and cause damage. By determining the association of the gut microbiome and its metabolites with various diseases, such as neurological disorders, future research will pave the way for the development of effective preventive and treatment modalities.
Keywords: Alzheimer’s disease; Autism spectrum disorder.; Gut microbiome; Parkinson’s disease; gut dysbiosis; gut metabolite; neurological disorders; therapeutic modalities.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures
Similar articles
-
Dysbiosis of the gut microbiota and its effect on α-synuclein and prion protein misfolding: consequences for neurodegeneration.Front Cell Infect Microbiol. 2024 Feb 16;14:1348279. doi: 10.3389/fcimb.2024.1348279. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38435303 Free PMC article. Review.
-
Dysbiosis of gut microbiota and microbial metabolites in Parkinson's Disease.Ageing Res Rev. 2018 Aug;45:53-61. doi: 10.1016/j.arr.2018.04.004. Epub 2018 Apr 26. Ageing Res Rev. 2018. PMID: 29705121 Review.
-
Gut-Brain Axis a Key Player to Control Gut Dysbiosis in Neurological Diseases.Mol Neurobiol. 2024 Dec;61(12):9873-9891. doi: 10.1007/s12035-023-03691-3. Epub 2023 Oct 18. Mol Neurobiol. 2024. PMID: 37851313 Review.
-
Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome.Int J Mol Sci. 2023 May 31;24(11):9577. doi: 10.3390/ijms24119577. Int J Mol Sci. 2023. PMID: 37298527 Free PMC article. Review.
-
The role of gut dysbiosis in Parkinson's disease: mechanistic insights and therapeutic options.Brain. 2021 Oct 22;144(9):2571-2593. doi: 10.1093/brain/awab156. Brain. 2021. PMID: 33856024 Review.
Cited by
-
Gut-brain axis and neurodegeneration: mechanisms and therapeutic potentials.Front Neurosci. 2024 Oct 23;18:1481390. doi: 10.3389/fnins.2024.1481390. eCollection 2024. Front Neurosci. 2024. PMID: 39513042 Free PMC article. Review.
-
Modulation of Gut Microbiome as a Therapeutic Modality for Auditory Disorders.Audiol Res. 2023 Oct 10;13(5):741-752. doi: 10.3390/audiolres13050066. Audiol Res. 2023. PMID: 37887847 Free PMC article. Review.
-
Intervention and research progress of gut microbiota-immune-nervous system in autism spectrum disorders among students.Front Microbiol. 2025 Mar 12;16:1535455. doi: 10.3389/fmicb.2025.1535455. eCollection 2025. Front Microbiol. 2025. PMID: 40143866 Free PMC article. Review.
-
Association between Mediterranean diet adherence and Parkinson's disease: a systematic review and meta-analysis.J Nutr Health Aging. 2025 Feb;29(2):100451. doi: 10.1016/j.jnha.2024.100451. Epub 2024 Dec 17. J Nutr Health Aging. 2025. PMID: 39693849 Free PMC article.
-
Causal relationship between gut microbiota, blood metabolites and autism spectrum disorder: a Mendelian randomization study.R Soc Open Sci. 2025 May 21;12(5):250158. doi: 10.1098/rsos.250158. eCollection 2025 May. R Soc Open Sci. 2025. PMID: 40400531 Free PMC article.
References
-
- Verhaar B.J.H., Hendriksen H.M.A., de Leeuw F.A., Doorduijn A.S., van Leeuwenstijn M., Teunissen C.E., Barkhof F., Scheltens P., Kraaij R., van Duijn C.M., Nieuwdorp M., Muller M., van der Flier W.M. Gut microbiota composition is related to AD pathology. Front. Immunol. 2022;12:794519. doi: 10.3389/fimmu.2021.794519. - DOI - PMC - PubMed
-
- Eshraghi R.S., Deth R.C., Mittal R., Aranke M., Kay S.I.S., Moshiree B., Eshraghi A.A. Early disruption of the microbiome leading to decreased antioxidant capacity and epigenetic changes: Implications for the rise in autism. Front. Cell. Neurosci. 2018;12:256. doi: 10.3389/fncel.2018.00256. - DOI - PMC - PubMed
-
- Kim C.H., Jung J., Lee Y., Kim K., Kang S., Kang G., Chu H., Kim S.Y., Lee S. Comparison of metabolites and gut microbes between patients with Parkinson’s disease and healthy individuals – a pilot clinical observational study (STROBE compliant). Healthcare (Basel) 2022;10(2):302. doi: 10.3390/healthcare10020302. - DOI - PMC - PubMed

