Age-related macular degeneration and myeloproliferative neoplasms - A common pathway
- PMID: 36200281
- PMCID: PMC9828081
- DOI: 10.1111/aos.15247
Age-related macular degeneration and myeloproliferative neoplasms - A common pathway
Abstract
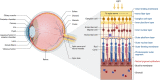
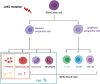
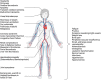
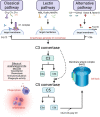
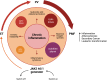
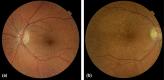
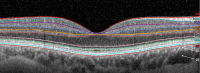
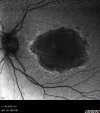
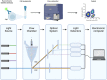
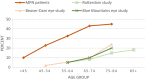
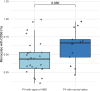
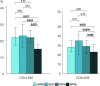
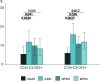
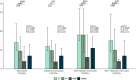
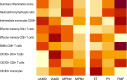
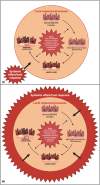
DANSK RESUMÉ (DANISH SUMMARY): Aldersrelateret makuladegeneration (AMD) er den hyppigste årsag til uopretteligt synstab og blindhed i højindkomstlande. Det er en progredierende nethindesygdom som gradvist fører til ødelaeggelse af de celler som er ansvarlige for vores centralsyn. De tidlige stadier er ofte asymptomatiske, imens senstadie AMD, som opdeles i to former, neovaskulaer AMD (nAMD) og geografisk atrofi (GA), begge udviser gradvist synstab, dog generelt med forskellig hastighed. Tidlig AMD er karakteriseret ved tilstedevaerelsen af druser og pigmentforandringer i nethinden mens nAMD og GA udviser henholdsvis karnydannelse i og atrofi af nethinden. AEtiologien er multifaktoriel og udover alder omfatter patogenesen miljø- og genetiske risikofaktorer. Forskning har specielt fokuseret på lokale forandringer i øjet hvor man har fundet at inflammation spiller en betydelig rolle for udviklingen af sygdommen, men flere studier tyder også på at systemiske forandringer og specielt systemisk inflammation spiller en vaesentlig rolle i patogenesen. De Philadelphia-negative myeloproliferative neoplasier (MPNs) er en gruppe af haematologiske kraeftsygdomme med en erhvervet genetisk defekt i den tidlige pluripotente stamcelle som medfører en overproduktion af en eller flere af blodets modne celler. Sygdommene er fundet at udvikle sig i et biologisk kontinuum fra tidligt cancerstadie, essentiel trombocytose (ET) over polycytaemi vera (PV) og endelig til det sene myelofibrose stadie (PMF). Symptomer hos disse patienter skyldes isaer den aendrede sammensaetning af blodet, hyperviskositet, kompromitteret mikrocirkulation og nedsat vaevsgennemblødning. Den øgede morbiditet og mortalitet beror i høj grad på tromboembolier, blødninger og leukemisk transformation. En raekke mutationer som driver MPN sygdommene er identificeret, bl.a. JAK2V617F-mutationen som medfører en deregulering JAK/STAT signalvejen, der bl.a. har betydning for cellers vaekst og overlevelse. Et tidligere stort registerstudie har vist at patienter med MPNs har en øget risiko for neovaskulaer AMD og et pilotstudie har vist øget forekomst af intermediaer AMD. Dette ønsker vi at undersøge naermere i et større studie i dette Ph.d.- projekt. Flere studier har også vist at kronisk inflammation spiller en vigtig rolle for både initiering og udvikling af den maligne celleklon hos MPNs og herfra er en "Human Inflammationsmodel" blevet udviklet. Siden er MPN sygdommene blevet anvendt som "model sygdomme" for en tilsvarende inflammationsmodel for udvikling af Alzheimers sygdom. I dette Ph.d.-projekt vil vi tilsvarende forsøge at undersøge systemisk inflammation i forhold til forekomst af druser. Det vil vi gøre ved at sammenligne systemiske immunologiske markører som tidligere er undersøgt hos patienter med AMD og sammenligne med MPN. Specielt er vi interesseret i systemiske immunologiske forskelle på patienter med MPN og druser (MPNd) og MPN med normale nethinder (MPNn). Denne afhandling består af to overordnede studier. I Studie I, undersøgte vi forekomsten af retinale forandringer associeret med AMD hos 200 patienter med MPN (artikel I). Studie II, omhandlede immunologiske ligheder ved AMD og MPN, og var opdelt i yderligere tre delstudier hvor vi undersøgte hhv. systemiske markører for inflammation, aldring og angiogenese (artikel II, III og IV). Vi undersøgte markørerne i fire typer af patienter: nAMD, intermediaer AMD (iAMD), MPNd og MPNn. Undersøgelsen af forskelle mellem MPNd og MPNn, vil gøre det muligt at identificere forandringer i immunsystemet som kunne vaere relevante for AMD-patogenesen. Vi vil endvidere sammenholde resultaterne for patienter med MPN med patienter som har iAMD og nAMD. I studie I (Artikel I) fandt vi at patienter med MPN har en signifikant højere praevalens af store druser og AMD tidligere i livet sammenlignet med estimater fra tre store befolkningsundersøgelser. Vi fandt også at forekomst af druser var associeret med højere neutrofil-lymfocyt ratio, hvilket indikerer et højere niveau af kronisk inflammation i patienterne med druser sammenlignet med dem uden druser. I studie II (Artikel II, III og IV) fandt vi flere immunologiske forskelle mellem patienter med MPNd og MPNn. Da vi undersøgte markører for inflammation, fandt vi en højere grad af systemisk inflammation i MPNd end MPNn. Dette blev vist ved en højere inflammationsscore (udregnet på baggrund af niveauer af pro-inflammatoriske markører), en højere neutrofil-lymfocyt ratio, samt indikationer på et dereguleret komplementsystem. Ved undersøgelse af aldringsmarkører fandt vi tegn på accelereret immunaldring hos MPNd i forhold til MPNn, hvilket kommer til udtryk ved en større procentdel af "effector memory T celler". Endelig fandt vi en vaesentlig lavere ekspression af CXCR3 på T celler og monocytter hos patienter med nAMD sammenlignet med iAMD, MPNd og MPNn. Dette er i overensstemmelse med tidligere studier hvor CXCR3 ekspression er fundet lavere end hos raske kontroller. Derudover fandt vi en faldende CXCR3 ekspression på monocytter over det biologiske MPN-kontinuum. Disse studier indikerer en involvering af CXCR3 i både nAMD og PMF, begge sygdomsstadier som er karakteriseret ved angiogenese og fibrose. Ud fra resultaterne af denne afhandling kan vi konkludere at forekomsten af druser og AMD hos MPN er øget i forhold til baggrundsbefolkningen. Endvidere viser vores resultater at systemisk inflammation muligvis spiller en vaesentlig større rolle i udviklingen af AMD end tidligere antaget. Vi foreslår derfor en AMD-model (Figur 18) hvor inflammation kan initiere og accelerere den normale aldersafhaengige akkumulation af affaldsstoffer i nethinden, som senere udvikler sig til druser, medførende øget lokal inflammation og med tiden tidlig og intermediaer AMD. Dette resulterer i den øgede risiko for udvikling til de invaliderende senstadier af AMD. ENGLISH SUMMARY: Age-related macular degeneration (AMD) is the most common cause of irreversible vision loss and blindness in high-income countries. It is a progressive retinal disease leading to damage of the cells responsible for central vision. The early stages of the disease are often asymptomatic, while late-stage AMD, which is divided into two entities, neovascular AMD and geographic atrophy (GA), both show vision loss, though generally with different progression rates. Drusen and pigmentary abnormalities in the retina characterise early AMD, while nAMD and GA show angiogenesis in and atrophy of the retina, respectively. The aetiology is multifactorial and, in addition to ageing, which is the most significant risk factor for developing AMD, environmental- and genetic risk factors are implicated in the pathogenesis. Research has focused on local changes in the eye where inflammation has been found to play an essential role, but studies also point to systemic alterations and especially systemic inflammation to be involved in the pathogenesis. The Philadelphia-negative myeloproliferative neoplasms (MPN) are a group of haematological cancers with an acquired genetic defect of the pluripotent haematopoietic stem cell, characterised by excess haematopoiesis of the myeloid cell lineage. The diseases have been found to evolve in a biological continuum from early cancer state, essential thrombocythemia, over polycythaemia vera (PV), to the advanced myelofibrosis stage (PMF). The symptoms in these patients are often a result of the changes in the blood composition, hyperviscosity, microvascular disturbances, and reduced tissue perfusion. The major causes of morbidity and mortality are thromboembolic- and haemorrhagic events, and leukemic transformation. A group of mutations that drive the MPNs has been identified, e.g., the JAK2V617F mutation, which results in deregulation of the JAK/STAT signal transduction pathway important, for instance, in cell differentiation and survival. A previous large register study has shown that patients with MPNs have an increased risk of neovascular AMD, and a pilot study has shown an increased prevalence of intermediate AMD. We wish to study this further in a larger scale study. Several studies have also shown that systemic inflammation plays an essential role in both the initiation and progression of the malignant cell clone in MPNs. From this knowledge, a "Human inflammation model" has been developed. Since then, the MPNs has been used as model diseases for a similar inflammation model for the development of Alzheimer's disease. In this PhD project, we would like to investigate systemic inflammation in relation to drusen presence. We will do this by comparing systemic immunological markers previously investigated in patients with AMD and compare with MPN. We are primarily interested in systemic immunological differences between patients with MPN and drusen (MPNd) and MPN with normal retinas (MPNn). This thesis consists of two main studies. Study I investigated the prevalence of retinal changes associated with AMD and the prevalence of different AMD stages in 200 patients with MPN (paper I). Study II examined immunological similarities between AMD and MPNs. This study was divided into three substudies exploring systemic markers of inflammation, ageing and angiogenesis, respectively. This was done in four types of patients: nAMD, intermediate AMD (iAMD), MPNd and MPNn. Investigating, differences between MPNd and MPNn, will make it possible to identify changes in the immune system, relevant for AMD pathogenesis. Additionally, we will compare patients with MPNs with patients with iAMD and nAMD. In study I (Paper I), we found that patients with MPNs have a significantly higher prevalence of large drusen and consequently AMD from an earlier age compared to the estimates from three large population-based studies. We also found that drusen prevalence was associated with a higher neutrophil-to-lymphocyte ratio indicating a higher level of chronic low-grade inflammation in patients with drusen compared to those without drusen. In study II (papers II, III and IV), we found immunological differences between patients with MPNd and MPNn. When we investigated markers of inflammation, we found a higher level of systemic inflammation in MPNd than MPNn. This was indicated by a higher inflammation score (based on levels of pro-inflammatory markers), a higher neutrophil-to-lymphocyte ratio, and indications of a deregulated complement system. When examining markers of ageing, we found signs of accelerated immune ageing in MPNd compared to MPNn, shown by more senescent effector memory T cells. Finally, when exploring a marker of angiogenesis, we found a lower CXCR3 expression on monocytes and T cells in nAMD compared to iAMD, MPNd and MPNn, in line with previous studies of nAMD compared to healthy controls. Further, we found decreasing CXCR3 expression over the MPN biological continuum. These studies indicate CXCR3 involvement in both nAMD and PMF, two disease stages characterised by angiogenesis and fibrosis. From the results of this PhD project, we can conclude that the prevalence of drusen and AMD is increased in patients with MPN compared to the general population. Further, our results show that systemic inflammation may play a far more essential role in AMD pathogenesis than previously anticipated. We, therefore, propose an AMD model (Figure 18) where inflammation can initiate and accelerate the normal age-dependent accumulation of debris in the retina, which later evolve into drusen, resulting in increased local inflammation, and over time early- and intermediate AMD. This results in the increased risk of developing the late debilitating stages of AMD.
© 2022 The Author. Acta Ophthalmologica published by John Wiley & Sons Ltd on behalf of Acta Ophthalmologica Scandinavica Foundation.
Figures


Similar articles
-
Mesenchymal stem cell therapy in aqueous deficient dry eye disease.Acta Ophthalmol. 2023 Oct;101 Suppl 277:3-27. doi: 10.1111/aos.15739. Acta Ophthalmol. 2023. PMID: 37840443 Review.
-
Ocular adnexal lymphoma: Subtype-specific clinical and genetic features.Acta Ophthalmol. 2022 Oct;100 Suppl 270:3-37. doi: 10.1111/aos.15248. Acta Ophthalmol. 2022. PMID: 36196757
-
Lower CXCR3 expression in both patients with neovascular AMD and advanced stages of chronic myeloproliferative blood cancers.PLoS One. 2022 Jun 16;17(6):e0269960. doi: 10.1371/journal.pone.0269960. eCollection 2022. PLoS One. 2022. PMID: 35709177 Free PMC article.
-
The microbiome seeding debate - let's frame it around women-centred care.Reprod Health. 2019 Jun 28;16(1):91. doi: 10.1186/s12978-019-0747-0. Reprod Health. 2019. PMID: 31253198 Free PMC article.
-
Psychedelic drugs in the treatment of anxiety, depression and addiction.Tidsskr Nor Laegeforen. 2018 Nov 12;138(18). doi: 10.4045/tidsskr.17.1110. Print 2018 Nov 13. Tidsskr Nor Laegeforen. 2018. PMID: 30421744 Review. English, Norwegian.
Cited by
-
Revisiting Circulating Extracellular Matrix Fragments as Disease Markers in Myelofibrosis and Related Neoplasms.Cancers (Basel). 2023 Aug 29;15(17):4323. doi: 10.3390/cancers15174323. Cancers (Basel). 2023. PMID: 37686599 Free PMC article. Review.
-
High Levels of C5a Are Associated With Reduced Macular Sensitivity in Patients With Myeloproliferative Neoplasms.Invest Ophthalmol Vis Sci. 2025 Feb 3;66(2):41. doi: 10.1167/iovs.66.2.41. Invest Ophthalmol Vis Sci. 2025. PMID: 39946135 Free PMC article.
References
-
- Alonso‐Fernández, P. , Puerto, M. , Maté, I. , Ribera, J.M. & De La Fuente, M. (2008) Neutrophils of centenarians show function levels similar to those of young adults. Journal of the American Geriatrics Society, 56, 2244–2251. - PubMed
-
- Ambati, J. , Ambati, B.K. , Yoo, S.H. , Ianchulev, S. & Adamis, A.P. (2003) Age‐related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Survey of Ophthalmology, 48, 257–293. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

