Modeling renal autoregulation in a hemodynamic, first-trimester gestational model
- PMID: 36200318
- PMCID: PMC9535437
- DOI: 10.14814/phy2.15484
Modeling renal autoregulation in a hemodynamic, first-trimester gestational model
Abstract
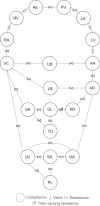
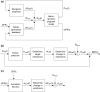
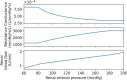
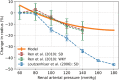
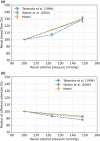
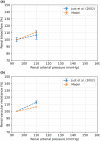
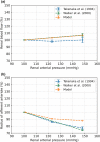
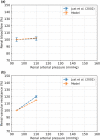
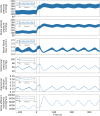
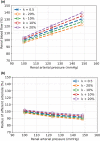
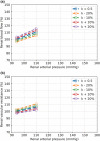
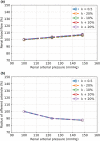
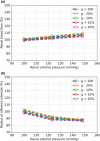
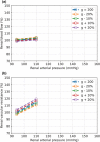
The maternal cardiovascular system, led by renal volume regulatory responses, changes during pregnancy to ensure an adequate circulation for fetal development and growth. Circulatory maladjustment predisposes to hypertensive complications during pregnancy. Mathematical models can be used to gain insight in the gestational cardiovascular physiology. In this study, we developed an accurate, robust, and transparent model for renal autoregulation implemented in an existing circulatory gestational model. This renal autoregulation model aims to maintain steady glomerular pressure by the myogenic response, and glomerular filtration rate by tubuloglomerular feedback, both by inducing a change in the radius, and thus resistance, of the afferent arteriole. The modeled response of renal blood flow and the afferent arteriole following blood pressure increase were compared to published observations in rats. With solely the myogenic response, our model had a maximum deviation of 7% in change in renal blood flow and 7% in renal vascular resistance. When both the myogenic response and tubuloglomerular feedback were concurrently activated, the maximum deviation was 7% in change in renal blood flow and 5% in renal vascular resistance. These results show that our model is able to represent renal autoregulatory behavior comparable to empirical data. Further studies should focus on extending the model with other regulatory mechanisms to understand the hemodynamic changes in healthy and complicated pregnancy.
Keywords: hemodynamic model; myogenic response; pregnancy; renal autoregulation; tubuloglomerular feedback.
© 2022 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Figures



Similar articles
-
A multinephron model of renal blood flow autoregulation by tubuloglomerular feedback and myogenic response.Acta Physiol Scand. 1991 Sep;143(1):71-92. doi: 10.1111/j.1748-1716.1991.tb09203.x. Acta Physiol Scand. 1991. PMID: 1957708
-
Aging Impairs Renal Autoregulation in Mice.Hypertension. 2020 Feb;75(2):405-412. doi: 10.1161/HYPERTENSIONAHA.119.13588. Epub 2019 Dec 16. Hypertension. 2020. PMID: 31838907 Free PMC article.
-
Molecular mechanisms of renal blood flow autoregulation.Curr Vasc Pharmacol. 2014;12(6):845-58. doi: 10.2174/15701611113116660149. Curr Vasc Pharmacol. 2014. PMID: 24066938 Free PMC article. Review.
-
Modeling the interaction between tubuloglomerular feedback and myogenic mechanisms in the control of glomerular mechanics.Front Physiol. 2024 Jun 20;15:1410764. doi: 10.3389/fphys.2024.1410764. eCollection 2024. Front Physiol. 2024. PMID: 38966231 Free PMC article.
-
Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms.Am J Physiol Regul Integr Comp Physiol. 2006 May;290(5):R1153-67. doi: 10.1152/ajpregu.00402.2005. Am J Physiol Regul Integr Comp Physiol. 2006. PMID: 16603656 Free PMC article. Review.
References
-
- Arnott, C. , Nelson, M. , Alfaro Ramirez, M. , Hyett, J. , Gale, M. , Henry, A. , Celermajer, D. S. , Taylor, L. , & Woodward, M. (2020). Maternal cardiovascular risk after hypertensive disorder of pregnancy. Heart, 106, 1927–1933. - PubMed
-
- Aukland, K. , & Oien, A. H. (1987). Renal autoregulation: Models combining tubuloglomerular feedback and myogenic response. The American Journal of Physiology, 252, F768–F783. - PubMed
-
- Avni, B. , Frenkel, G. , Shahar, L. , Golik, A. , Sherman, D. , & Dishy, V. (2010). Aortic stiffness in normal and hypertensive pregnancy. Blood Pressure, 19, 11–15. - PubMed
-
- Baylis, C. (1994). Glomerular filtration and volume regulation in gravid animal models. Bailliere's Clinical Obstetrics and Gynaecology, 8, 235–264. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

