Oral HDAC inhibitor tucidinostat in patients with relapsed or refractory peripheral T-cell lymphoma: phase IIb results
- PMID: 36200417
- PMCID: PMC9973490
- DOI: 10.3324/haematol.2022.280996
Oral HDAC inhibitor tucidinostat in patients with relapsed or refractory peripheral T-cell lymphoma: phase IIb results
Abstract
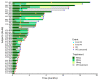
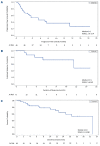
Tucidinostat (formerly known as chidamide) is an orally available, novel benzamide class of histone deacetylase (HDAC) inhibitor that selectively blocks class I and class IIb HDAC. This multicenter phase IIb study aimed to investigate the efficacy and safety of tucidinostat, 40 mg twice per week (BIW), in patients with relapsed/refractory (R/R) peripheral T-cell lymphoma (PTCL). The primary endpoint was overall response rate (ORR) assessed by an independent overall efficacy review committee. Between March 2017 and March 2019, 55 patients were treated, and 46 and 55 were evaluated for efficacy and safety, respectively. Twenty-one of 46 patients achieved objective responses with an ORR of 46% (95% confidence interval : 30.9-61.0), including five patients with complete response (CR). Responses were observed across various PTCL subtypes. In angioimmunoblastic T-cell lymphoma, there were two CR and five partial responses (PR) among eight patients, achieving an ORR of 88%. The disease control rate (CR + PR + stable disease) was 72% (33/46). The median progression-free survival, duration of response, and overall survival were 5.6 months, 11.5 months, 22.8 months, respectively. The most common adverse events (AE) (all grades) were thrombocytopenia, neutropenia, leukopenia, anemia, and diarrhea. The grade ≥3 AE emerging in ≥20% of patients included thrombocytopenia (51%), neutropenia (36%), lymphopenia (22%), and leukopenia (20%). Importantly, most of the AE were manageable by supportive care and dose modification. In conclusion, the favorable efficacy and safety profiles indicate that tucidinostat could be a new therapeutic option in patients with R/R PTCL (clinicaltrials gov. Identifier: NCT02953652).
Figures

References
-
- Horwitz SM, Zelenetz AD, Gordon LI, et al. . NCCN guidelines insights: non-Hodgkin's lymphomas, Version 3.2016. J Natl Compr Canc Netw. 2016;14(9):1067-1079. - PubMed
-
- d'Amore F, Gaulard P, Trümper L, et al. . Peripheral T-cell lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015(Suppl 5):v108-115. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

