Lenalidomide-based triplet regimens in first relapsed multiple myeloma patients: real-world evidence from a propensity score matched analysis
- PMID: 36200419
- PMCID: PMC9973473
- DOI: 10.3324/haematol.2022.281342
Lenalidomide-based triplet regimens in first relapsed multiple myeloma patients: real-world evidence from a propensity score matched analysis
Abstract
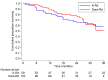
Lenalidomide and dexamethasone (Rd)-based triplets, in particular carfilzomib-Rd (KRd) and daratumumab-Rd (DaraRd), represent a standard of care in lenalidomide-sensitive multiple myeloma (MM) patients in first relapse. Meta-analysis of randomized clinical trials (RCT), suggested better outcome with DaraRd. Trying to address this issue in clinical practice, we collected data of 430 consecutive MM patients addressed to Rd-based triplets in first relapse between January 2017 and March 2021. Overall, the most common used regimen was DaraRd, chosen in almost half of the cases (54.4%), followed by KRd (34.6%). Different triplets were used much less commonly. In an attempt to limit the imbalance of a retrospective analysis, we conducted a propensity score matching (PSM) comparison between DaraRd and KRd. After PSM, efficacy of DaraRd versus KRd was similar in terms of overall-response rate (ORR) (OR: 0.9, P=0.685) as well as of very good partial response (VGPR) or better (OR: 0.9, P=0.582). The median progression-free survival (PFS) was significantly longer for DaraRd (29.8 vs. 22.5 months; P=0.028). DaraRd was tolerated better, registering a lower rate of grade 3-4 non-hematological toxicity (OR: 0.4, P<0.001). With the limitations of any retrospective analysis, our real-life PSM comparison between DaraRd and KRd, in first-relapse MM patients, showed better tolerability and prolonged PFS of DaraRd, although with some gaps of performance, in particular of DaraRd, with respect to RCT. Carfilzomib-containing regimens, like KRd, still remain a valid second-line option in the emerging scenario of first-line daratumumab-based therapy.
Figures



References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046-1060. - PubMed
-
- Harousseau JL, Attal M. How I treat first relapse of myeloma. Blood. 2017;130(8):963-973. - PubMed
-
- Kastritis E, Terpos E, Dimopoulos MA. How I treat relapsed multiple myeloma. Blood. 2022;139(19):2904-2917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

