Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses
- PMID: 36200477
- PMCID: PMC10092593
- DOI: 10.1111/dom.14890
Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses
Abstract
Aims: Evaluate the effects of once-weekly subcutaneous semaglutide 2.4 mg on cardiometabolic risk factors in people with overweight/obesity without diabetes in the STEP 1 and 4 trials.
Materials and methods: STEP 1 and 4 were phase III, 68-week, placebo-controlled trials of once-weekly semaglutide 2.4 mg combined with lifestyle intervention; STEP 4 had a 20-week semaglutide run-in and 48-week randomized withdrawal period. Participants had a body mass index ≥30 kg/m2 or ≥27 kg/m2 with one or more weight-related comorbidity, without diabetes. Pre-specified endpoints were changes in waist circumference, systolic/diastolic blood pressure (SBP/DBP), lipids, fasting plasma glucose (FPG), fasting serum insulin and antihypertensive/lipid-lowering medication use. Post-hoc assessments included non-high-density lipoprotein (HDL) cholesterol, homeostatic model assessment of insulin resistance (HOMA-IR; STEP 1 only), atherosclerotic cardiovascular disease (ASCVD) risk (American College of Cardiology/American Heart Association algorithm; STEP 1 only) and cardiometabolic risk factors by weight loss achieved (<5%, 5% to <10%, 10% to <15%, or ≥15%) (STEP 1 only).
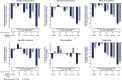
Results: Of the 1961 participants in STEP 1 and 803 in STEP 4, most had one or more complication/comorbidity at baseline, with dyslipidaemia and hypertension most prevalent. In STEP 1, reductions in waist circumference, SBP, DBP, FPG, fasting serum insulin, lipids and HOMA-IR were greater with semaglutide versus placebo (p ≤ .001). Reductions in SBP, non-HDL cholesterol, low-density lipoprotein cholesterol and FPG were generally greater with semaglutide than placebo within weight-loss categories. Non-significant ASCVD risk reductions were observed with semaglutide versus placebo (p > .05). In STEP 4, improvements in waist circumference, SBP, FPG, fasting serum insulin and lipids during the semaglutide run-in (week 0-20) were maintained over week 20-68 with continued semaglutide, but deteriorated following the switch to placebo (p < .001 [week 20-68]). Net reductions in antihypertensive/lipid-lowering medication use occurred with semaglutide versus placebo (both trials).
Conclusions: Semaglutide may improve cardiometabolic risk factors and reduce antihypertensive/lipid-lowering medication use versus placebo in adults with overweight/obesity without diabetes. These potential benefits were not maintained after treatment discontinuation.
Gov numbers: STEP 1 NCT03548935, STEP 4 NCT03548987.
Keywords: GLP-1 analogue; cardiovascular disease; obesity therapy; randomized trial; weight control.
© 2022 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
MB is an employee of Novo Nordisk A/S. MD has received research funding from AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk and Sanofi‐Aventis, paid to her institution; has acted as a consultant, advisory board member, and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi‐Aventis; an advisory board member and speaker for AstraZeneca; an advisory board member for Gilead Sciences Ltd and Lexicon; and a speaker for Napp Pharmaceuticals and Takeda Pharmaceuticals International Inc. She is co‐funded by the NIHR Leicester Biomedical Research Centre. JED has received honoraria, speaker, or other fees from Aegerion Pharmaceuticals, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi‐Aventis and Takeda Pharmaceuticals International Inc.; has acted as a consultant for GENinCode. He has unpaid leadership or fiduciary roles in Our Future Health and Public Health England. WTG has served as a volunteer on advisory boards, without receipt of financial compensation, for Boehringer Ingelheim, Eli Lilly, JAZZ Pharmaceuticals, Novo Nordisk and Pfizer, and served on advisory boards for Alnylam Pharmaceuticals and Fractyl Health, where he received financial compensation for this service. He has participated as site principal investigator for multicentred clinical trials sponsored by his university and funded by Eli Lilly, Epitomee, Novo Nordisk, and Pfizer. UK was an employee of Novo Nordisk A/S during the conduct of the trials. MNK has received research grants from AstraZeneca and Boehringer Ingelheim; has served as a consultant/advisory board member for Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Sanofi and Vifor Pharma; has received honoraria from AstraZeneca, Boehringer Ingelheim and Novo Nordisk. RK has received grants and speaker fees from, and served as an advisory board member for, Novo Nordisk; and has received honoraria from CME Outfitters, Medscape, Pri‐Med, Rockpointe and Vindico Medical Education. DMR has received research grants, consultancy fees, travel fees and honoraria from, acted as an advisory board member, speaker, and principal investigator for Novo Nordisk; has received research grants from, and is a principal investigator and advisor for Boehringer Ingelheim; has received research funds from Epitomee Medical; and has received honoraria from the Endocrine Society, Medscape and the PeerView Institute. SV has received research grants and/or contracts, honoraria, and consulting fees from, and acted as an advisory board member for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Novo Nordisk; has received research grants and/or contracts and honoraria from, and acted as an advisory board member for Amarin, Bayer, HLS Therapeutics, Janssen and Novartis; has received research grants and/or contracts from, and acted as an advisory board member for Amgen; has received research grants and/or contracts and honoraria from PhaseBio, Pfizer and Sanofi; has received research grants and/or contracts from Bristol‐Myers Squibb and Otsuka; has received honoraria from the Canadian Medical & Surgical Knowledge Translation Research Group, EOCI Pharmacomm Ltd, Sun Pharmaceuticals and Toronto Knowledge Translation Working Group. He is also the President of the Canadian Medical and Surgical Knowledge Translation Research Group and holds the Tier 1 Canada Research Chair in Cardiovascular Surgery. NZ is an employee and shareholder of Novo Nordisk A/S.
Figures

References
-
- Bessesen DH, Van Gaal LF. Progress and challenges in anti‐obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6(3):237‐248. - PubMed
-
- Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715‐723. - PubMed
-
- Kachur S, Lavie CJ, de Schutter A, Milani RV, Ventura HO. Obesity and cardiovascular diseases. Minerva Med. 2017;108(3):212‐228. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

