Evolutionary consequences of vector-borne transmission: how using vectors shapes host, vector and pathogen evolution
- PMID: 36200511
- PMCID: PMC10090782
- DOI: 10.1017/S0031182022001378
Evolutionary consequences of vector-borne transmission: how using vectors shapes host, vector and pathogen evolution
Abstract
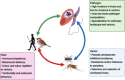
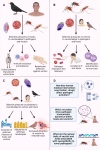
Transmission mode is a key factor that influences host–parasite coevolution. Vector-borne pathogens are among the most important disease agents for humans and wildlife due to their broad distribution, high diversity, prevalence and lethality. They comprise some of the most important and widespread human pathogens, such as yellow fever, leishmania and malaria. Vector-borne parasites (in this review, those transmitted by blood-feeding Diptera) follow unique transmission routes towards their vertebrate hosts. Consequently, each part of this tri-partite (i.e. parasite, vector and host) interaction can influence co- and counter-evolutionary pressures among antagonists. This mode of transmission may favour the evolution of greater virulence to the vertebrate host; however, pathogen–vector interactions can also have a broad spectrum of fitness costs to the insect vector. To complete their life cycle, vector-borne pathogens must overcome immune responses from 2 unrelated organisms, since they can activate responses in both vertebrate and invertebrate hosts, possibly creating a trade-off between investments against both types of immunity. Here, we assess how dipteran vector-borne transmission shapes the evolution of hosts, vectors and the pathogens themselves. Hosts, vectors and pathogens co-evolve together in a constant antagonistic arms race with each participant's primary goal being to maximize its performance and fitness.
Keywords: Coevolution; evolution; parasite–host interaction; vector ecology; vector-borne diseases; virulence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The role of the ratio of vector and host densities in the evolution of transmission modes in vector-borne diseases. The example of sylvatic Trypanosoma cruzi.J Theor Biol. 2012 Nov 7;312:133-42. doi: 10.1016/j.jtbi.2012.07.028. Epub 2012 Aug 7. J Theor Biol. 2012. PMID: 22892441
-
Alice in microbes' land: adaptations and counter-adaptations of vector-borne parasitic protozoa and their hosts.FEMS Microbiol Rev. 2016 Sep;40(5):664-85. doi: 10.1093/femsre/fuw018. Epub 2016 Jul 10. FEMS Microbiol Rev. 2016. PMID: 27400870 Review.
-
Vector-Borne Pathogen and Host Evolution in a Structured Immuno-Epidemiological System.Bull Math Biol. 2017 Feb;79(2):325-355. doi: 10.1007/s11538-016-0239-0. Epub 2016 Dec 28. Bull Math Biol. 2017. PMID: 28032207
-
Role of Virulence Factors of Trypanosomatids in the Insect Vector and Putative Genetic Events Involved in Surface Protein Diversity.Front Cell Infect Microbiol. 2022 Apr 28;12:807172. doi: 10.3389/fcimb.2022.807172. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35573777 Free PMC article. Review.
-
Interactions between insect vectors and plant pathogens span the parasitism-mutualism continuum.Biol Lett. 2023 Mar;19(3):20220453. doi: 10.1098/rsbl.2022.0453. Epub 2023 Mar 8. Biol Lett. 2023. PMID: 36883313 Free PMC article.
Cited by
-
Hard Ticks as Vectors: The Emerging Threat of Tick-Borne Diseases in India.Pathogens. 2024 Jul 2;13(7):556. doi: 10.3390/pathogens13070556. Pathogens. 2024. PMID: 39057783 Free PMC article. Review.
-
The impact of blood on vector-borne diseases with emphasis on mosquitoes and sand flies.Trends Parasitol. 2025 Mar;41(3):196-209. doi: 10.1016/j.pt.2025.01.009. Epub 2025 Feb 19. Trends Parasitol. 2025. PMID: 39979193 Review.
-
N6-methyltubercidin gives sterile cure in a cutaneous Leishmania amazonensis mouse model.Parasitology. 2024 Apr;151(5):506-513. doi: 10.1017/S0031182024000362. Epub 2024 Mar 27. Parasitology. 2024. PMID: 38533610 Free PMC article.
-
Haemosporidian infection risk and community structure determined by duck feeding guild.Parasitology. 2025 Feb;152(2):217-228. doi: 10.1017/S0031182025000137. Parasitology. 2025. PMID: 39957516 Free PMC article.
-
Cell surface Toll-like receptor polymorphisms influence Bartonella and ectoparasite infections in striped hamsters.iScience. 2025 Jun 13;28(7):112883. doi: 10.1016/j.isci.2025.112883. eCollection 2025 Jul 18. iScience. 2025. PMID: 40678527 Free PMC article.
References
-
- Alizon S and van Baalen M (2008) Transmission-virulence trade-offs in vector-borne diseases. Theoretical Population Biology 74, 6–15. - PubMed
-
- Atkinson CT, Saili KS, Utzurrum RB and Jarvi SI (2013) Experimental evidence for evolved tolerance to avian malaria in a wild population of low elevation Hawai'i ‘amakihi (Hemignathus virens). EcoHealth 10, 366–375. - PubMed
-
- Atkinson CT, Utzurrum RB, Lapointe DA, Camp RJ, Crampton LH, Foster JT and Giambelluca TW (2014) Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands – an ongoing conservation crisis on the island of Kaua'i. Global Change Biology 20, 2426–2436. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

