Antiproliferative Activity of (-)-Isopulegol-based 1,3-Oxazine, 1,3-Thiazine and 2,4-Diaminopyrimidine Derivatives
- PMID: 36200514
- PMCID: PMC9535514
- DOI: 10.1002/open.202200169
Antiproliferative Activity of (-)-Isopulegol-based 1,3-Oxazine, 1,3-Thiazine and 2,4-Diaminopyrimidine Derivatives
Abstract
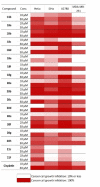
A series of novel heterocyclic structures, namely 1,3-oxazines, 1,3-thiazines and 2,4-diaminopyrimidines, were designed and synthesised. The bioassay tests demonstrated that, among these analogues, 2,4-diaminopyridine derivatives showed significant antiproliferative activity against different human cancer cell lines (A2780, SiHa, HeLa, MCF-7 and MDA-MB-231). Pyrimidines substituted with N2 -(p-trifluoromethyl)aniline, in particular, displayed a potent inhibitory effect on the growth of cancer cells. Structure-activity relationships were also studied from the aspects of stereochemistry on the aminodiol moiety as well as exploring the effects of substituents on the pyrimidine scaffold.
Keywords: (+)-neoisopulegol; (−)-isopulegol; 1,3-oxazines; 1,3-thiazines; 2,4-diaminopyrimidines.
© 2022 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
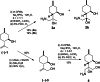
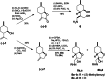
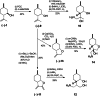
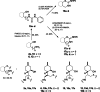


References
-
- Aiello A., Fattorusso E., Giordano A., Menna M., Navarrete C., Muñoz E., Tetrahedron 2009, 65, 4384–4388.
-
- Vijai Kumar Reddy T., Jyotsna A., Prabhavathi Devi B. L. A., Prasad R. B. N., Poornachandra Y., Ganesh Kumar C., Eur. J. Med. Chem. 2016, 120, 86–96. - PubMed
-
- Guasch J., Giménez-Nueno I., Funes-Ardoiz I., Bernús M., Matheu M. I., Maseras F., Castillón S., Díaz Y., Chem. Eur. J. 2018, 24, 4635–4642. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

