Scalable Microreactor Concept for the Continuous Kolbe Electrolysis of Carboxylic Acids Using Aqueous Electrolyte
- PMID: 36200517
- PMCID: PMC9535501
- DOI: 10.1002/open.202200171
Scalable Microreactor Concept for the Continuous Kolbe Electrolysis of Carboxylic Acids Using Aqueous Electrolyte
Abstract
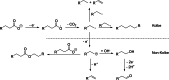
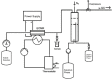
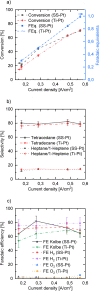
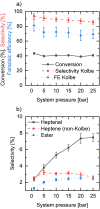
The Kolbe electrolysis is a promising reaction to combine the usage of electrons as reagents and the application of renewable generated carboxylic acids as raw materials producing value added chemicals. Within this study, the electrolysis was conducted in a specially developed concept electrochemical microreactor and draws the particular attention to continuous operation and reuse of the aqueous electrolyte as well as of the dissolved unreacted feedstock. The electrolysis was conducted in alkaline aqueous solution using n-octanoic acid as model substance. Platinized titanium as anode material in an undivided cell setup was shown to give Kolbe selectivity above 90 %. During the technically relevant conditions of current densities up to 0.6 A cm-2 and overall electrolysis times of up to 3 h, a high electrode stability was observed. Finally, a proof-of-concept continuous operation and the numbering up potential of the ECMR could be demonstrated.
© 2022 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Platinized Titanium as Alternative Cost-Effective Anode for Efficient Kolbe Electrolysis in Aqueous Electrolyte Solutions.ChemSusChem. 2021 Aug 9;14(15):3097-3109. doi: 10.1002/cssc.202100854. Epub 2021 Jul 3. ChemSusChem. 2021. PMID: 34060244 Free PMC article.
-
Beyond Kolbe and Hofer-Moest: Electrochemical Synthesis of Carboxylic Anhydrides from Carboxylic Acids.ChemistryOpen. 2022 May;11(5):e202200059. doi: 10.1002/open.202200059. ChemistryOpen. 2022. PMID: 35561027 Free PMC article.
-
On-line Electrode Dissolution Monitoring during Organic Electrosynthesis: Direct Evidence of Electrode Dissolution during Kolbe Electrolysis.ChemSusChem. 2022 Mar 8;15(5):e202102228. doi: 10.1002/cssc.202102228. Epub 2022 Feb 3. ChemSusChem. 2022. PMID: 35114080 Free PMC article.
-
Towards Versatile and Sustainable Hydrogen Production through Electrocatalytic Water Splitting: Electrolyte Engineering.ChemSusChem. 2017 Apr 10;10(7):1318-1336. doi: 10.1002/cssc.201601583. Epub 2017 Mar 9. ChemSusChem. 2017. PMID: 27984671 Free PMC article. Review.
-
High-Temperature CO2 Electrolysis in Solid Oxide Electrolysis Cells: Developments, Challenges, and Prospects.Adv Mater. 2019 Dec;31(50):e1902033. doi: 10.1002/adma.201902033. Epub 2019 Jul 7. Adv Mater. 2019. PMID: 31282069 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

