Continuous-Flow Divergent Lithiation of 2,3-Dihalopyridines: Deprotolithiation versus Halogen Dance
- PMID: 36200571
- PMCID: PMC10092453
- DOI: 10.1002/chem.202202286
Continuous-Flow Divergent Lithiation of 2,3-Dihalopyridines: Deprotolithiation versus Halogen Dance
Abstract
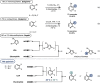
We describe herein the first halogen dance (HD) in continuous flow on 2-chloro-3-bromopyridine by selectively trapping a (pyridin-4-yl)lithium species that is known to undergo the halogen-dance process. In addition, this lithiated intermediate was trapped at lower temperature before the HD occurs. The HD process was extended to fluoro-iodopyridines by using various electrophiles to afford 28 examples with yields ranging from 42 to 97 % with very short residence times. Finally, scale up of the reaction was demonstrated, affording a promising space-time yield (STY) of 4.2 kg.h-1 .L-1 .
Keywords: continuous flow; flash chemistry; halogen dance; lithiation; pyridines.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
5-Bromo-2-chloro-4-fluoro-3-iodopyridine as a Halogen-rich Intermediate for the Synthesis of Pentasubstituted Pyridines.J Org Chem. 2022 Mar 4;87(5):2559-2568. doi: 10.1021/acs.joc.1c02507. Epub 2022 Jan 12. J Org Chem. 2022. PMID: 35020387
-
Lithiation of prochiral 2,2'-dichloro-5,5'-dibromo-4,4'-bipyridine as a tool for the synthesis of chiral polyhalogenated 4,4'-bipyridines.J Org Chem. 2013 Aug 2;78(15):7683-9. doi: 10.1021/jo401255q. Epub 2013 Jul 24. J Org Chem. 2013. PMID: 23837459
-
Long-Range Halogen Dance Reaction in 4,5-Dihalogeno-2-(Sulfur-Containing Heterocyclyl)thiazole.Chem Pharm Bull (Tokyo). 2024;72(12):1061-1064. doi: 10.1248/cpb.c24-00561. Chem Pharm Bull (Tokyo). 2024. PMID: 39675969
-
Dancing for Healthy Aging: Functional and Metabolic Perspectives.Altern Ther Health Med. 2019 Jan;25(1):44-63. Altern Ther Health Med. 2019. PMID: 29428927 Review.
-
Halogens in Protein-Ligand Binding Mechanism: A Structural Perspective.J Med Chem. 2019 Nov 14;62(21):9341-9356. doi: 10.1021/acs.jmedchem.8b01453. Epub 2019 May 28. J Med Chem. 2019. PMID: 31117513 Review.
Cited by
-
Continuous flow synthesis enabling reaction discovery.Chem Sci. 2024 Feb 28;15(13):4618-4630. doi: 10.1039/d3sc06808k. eCollection 2024 Mar 27. Chem Sci. 2024. PMID: 38550700 Free PMC article. Review.
References
-
- None
-
- El-Sayed H. A., Moustafa A. H., El-Torky A. E., Abd El-Salam E. A., Russ. J. Gen. Chem. 2017, 87, 2401–2408;
-
- Gerloff J., Mignot A., Barth H., Heintze K., Eur. J. Clin. Pharmacol. 1996, 50, 293–297; - PubMed
-
- Zou H. Y., Li Q., Lee J. H., Arango M. E., McDonnell S. R., Yamazaki S., Koudriakova T. B., Alton G., Cui J. J., Kung P.-P., Nambu M. D., Los G., Bender S. L., Mrockowski B., Christensen J. G., Cancer Res. 2007, 67, 4408–4417; - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

