Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment
- PMID: 36200701
- PMCID: PMC9619622
- DOI: 10.1093/cid/ciac663
Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment
Abstract
Background: Nirmatrelvir/ritonavir, the first severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) protease inhibitor, reduces the risk of hospitalization and death by coronavirus disease 2019 (COVID-19) but has been associated with symptomatic rebound after therapy completion.
Methods: Six individuals with relapse of COVID-19 symptoms after treatment with nirmatrelvir/ritonavir, 2 individuals with rebound symptoms without prior antiviral therapy and 7 patients with acute Omicron infection (controls) were studied. Soluble biomarkers and serum SARS-CoV-2 nucleocapsid protein were measured. Nasal swabs positive for SARS-CoV-2 underwent viral isolation and targeted viral sequencing. SARS-CoV-2 anti-spike, anti-receptor-binding domain, and anti-nucleocapsid antibodies were measured. Surrogate viral neutralization tests against wild-type and Omicron spike protein, as well as T-cell stimulation assays, were performed.
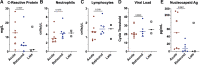
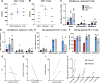
Results: High levels of SARS-CoV-2 anti-spike immunoglobulin G (IgG) antibodies were found in all participants. Anti-nucleocapsid IgG and Omicron-specific neutralizing antibodies increased in patients with rebound. Robust SARS-CoV-2-specific T-cell responses were observed, higher in rebound compared with early acute COVID-19 patients. Inflammatory markers mostly decreased during rebound. Two patients sampled longitudinally demonstrated an increase in activated cytokine-producing CD4+ T cells against viral proteins. No characteristic resistance mutations were identified. SARS-CoV-2 was isolated by culture from 1 of 8 rebound patients; Polybrene addition increased this to 5 of 8.
Conclusions: Nirmatrelvir/ritonavir treatment does not impede adaptive immune responses to SARS-CoV-2. Clinical rebound corresponds to development of a robust antibody and T-cell immune response, arguing against a high risk of disease progression. The presence of infectious virus supports the need for isolation and assessment of longer treatment courses.
Clinical trials registration: NCT04401436.
Keywords: COVID-19; COVID-19 rebound; COVID-19 transmission; antiviral therapy; nirmatrelvir/ritonavir.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2022.
Conflict of interest statement
Potential conflicts of interest. E. G. reports grants or contracts from the National Science Foundation and NIH and a leadership or fiduciary role in other board, society, committee, or advocacy group for American Society for Microbiology (ASM, unpaid). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



Update of
-
COVID-19 redux: clinical, virologic, and immunologic evaluation of clinical rebound after nirmatrelvir/ritonavir.medRxiv [Preprint]. 2022 Jun 17:2022.06.16.22276392. doi: 10.1101/2022.06.16.22276392. medRxiv. 2022. Update in: Clin Infect Dis. 2023 Feb 18;76(4):573-581. doi: 10.1093/cid/ciac663. PMID: 35734093 Free PMC article. Updated. Preprint.
References
-
- Center for Drug Evaluation and Research (CDER) . Emergency Use Authorization (EUA) for Paxlovid (nirmatrelvir tablets co-packaged with ritonavir tablets) Center for Drug Evaluation and Research (CDER) Review. Silver Spring, MD: Food and Drug Administration, 2021. Available at: https://www.fda.gov/media/155194/download. Accessed 27 August 2022.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

