Hot-Carrier Transfer across a Nanoparticle-Molecule Junction: The Importance of Orbital Hybridization and Level Alignment
- PMID: 36200744
- PMCID: PMC9650767
- DOI: 10.1021/acs.nanolett.2c02327
Hot-Carrier Transfer across a Nanoparticle-Molecule Junction: The Importance of Orbital Hybridization and Level Alignment
Abstract
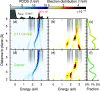
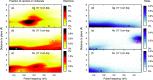
While direct hot-carrier transfer can increase photocatalytic activity, it is difficult to discern experimentally and competes with several other mechanisms. To shed light on these aspects, here, we model from first-principles hot-carrier generation across the interface between plasmonic nanoparticles and a CO molecule. The hot-electron transfer probability depends nonmonotonically on the nanoparticle-molecule distance and can be effective at long distances, even before a strong chemical bond can form; hot-hole transfer on the other hand is limited to shorter distances. These observations can be explained by the energetic alignment between molecular and nanoparticle states as well as the excitation frequency. The hybridization of the molecular orbitals is the key predictor for hot-carrier transfer in these systems, emphasizing the necessity of ground state hybridization for accurate predictions. Finally, we show a nontrivial dependence of the hot-carrier distribution on the excitation energy, which could be exploited when optimizing photocatalytic systems.
Keywords: Adsorption; Hot-carrier; Nanoparticles; Plasmonic catalysis; TDDFT.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Direct hot-carrier transfer in plasmonic catalysis.Faraday Discuss. 2019 May 1;214:189-197. doi: 10.1039/c8fd00154e. Epub 2019 Mar 11. Faraday Discuss. 2019. PMID: 30855061
-
Hot carrier generation in a strongly coupled molecule-plasmonic nanoparticle system.Nanophotonics. 2023 Mar 15;12(9):1711-1722. doi: 10.1515/nanoph-2022-0700. eCollection 2023 Apr. Nanophotonics. 2023. PMID: 39634110 Free PMC article.
-
Quantifying Wavelength-Dependent Plasmonic Hot Carrier Energy Distributions at Metal/Semiconductor Interfaces.ACS Nano. 2019 Mar 26;13(3):3629-3637. doi: 10.1021/acsnano.9b00219. Epub 2019 Mar 1. ACS Nano. 2019. PMID: 30807695
-
Plasmonic photocatalysis.Rep Prog Phys. 2013 Apr;76(4):046401. doi: 10.1088/0034-4885/76/4/046401. Epub 2013 Mar 4. Rep Prog Phys. 2013. PMID: 23455654 Review.
-
Resonance energy transfer from a fluorescent dye to a metal nanoparticle.J Chem Phys. 2006 Nov 14;125(18):181102. doi: 10.1063/1.2400037. J Chem Phys. 2006. PMID: 17115730 Review.
Cited by
-
Computational Discovery of Design Principles for Plasmon-Driven Bond Activation on Alloy Antenna Reactors.ACS Nano. 2025 Mar 18;19(10):9860-9867. doi: 10.1021/acsnano.4c13602. Epub 2025 Mar 7. ACS Nano. 2025. PMID: 40052953 Free PMC article.
-
Tailoring Hot-Carrier Distributions of Plasmonic Nanostructures through Surface Alloying.ACS Nano. 2024 Feb 27;18(8):6398-6405. doi: 10.1021/acsnano.3c11418. Epub 2024 Feb 16. ACS Nano. 2024. PMID: 38363179 Free PMC article.
-
Advances in fundamentals and application of plasmon-assisted CO2 photoreduction.Nanophotonics. 2024 Feb 1;13(4):387-417. doi: 10.1515/nanoph-2023-0793. eCollection 2024 Feb. Nanophotonics. 2024. PMID: 39635649 Free PMC article. Review.
-
Plasmonic Hot-Carrier Engineering at Bimetallic Nanoparticle/Semiconductor Interfaces: A Computational Perspective.Small. 2025 Mar;21(11):e2410173. doi: 10.1002/smll.202410173. Epub 2025 Feb 16. Small. 2025. PMID: 39955760 Free PMC article.
-
Recent Advances in Real-Time Time-Dependent Density Functional Theory Simulations of Plasmonic Nanostructures and Plasmonic Photocatalysis.ACS Nanosci Au. 2023 May 19;3(4):269-279. doi: 10.1021/acsnanoscienceau.2c00061. eCollection 2023 Aug 16. ACS Nanosci Au. 2023. PMID: 37601917 Free PMC article. Review.
References
-
- Nugroho F. A. A.; Darmadi I.; Cusinato L.; Susarrey-Arce A.; Schreuders H.; Bannenberg L. J.; da Silva Fanta A. B.; Kadkhodazadeh S.; Wagner J. B.; Antosiewicz T. J.; Hellman A.; Zhdanov V. P.; Dam B.; Langhammer C. Metal–Polymer Hybrid Nanomaterials for Plasmonic Ultrafast Hydrogen Detection. Nat. Mater. 2019, 18, 489–495. 10.1038/s41563-019-0325-4. - DOI - PubMed
-
- Darmadi I.; Khairunnisa S. Z.; Tomeček D.; Langhammer C. Optimization of the Composition of PdAuCu Ternary Alloy Nanoparticles for Plasmonic Hydrogen Sensing. ACS Applied Nano Materials 2021, 4, 8716–8722. 10.1021/acsanm.1c01242. - DOI
-
- Aslam U.; Rao V. G.; Chavez S.; Linic S. Catalytic Conversion of Solar to Chemical Energy on Plasmonic Metal Nanostructures. Nature Catalysis 2018, 1, 656–665. 10.1038/s41929-018-0138-x. - DOI
-
- Li R.; Cheng W.-H.; Richter M. H.; DuChene J. S.; Tian W.; Li C.; Atwater H. A. Unassisted Highly Selective Gas-Phase CO2 Reduction with a Plasmonic Au/p-GaN Photocatalyst Using H2O as an Electron Donor. ACS Energy Letters 2021, 6, 1849–1856. 10.1021/acsenergylett.1c00392. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

