Role of Bifidobacterium pseudocatenulatum in Degradation and Consumption of Xylan-Derived Carbohydrates
- PMID: 36200766
- PMCID: PMC9599329
- DOI: 10.1128/aem.01299-22
Role of Bifidobacterium pseudocatenulatum in Degradation and Consumption of Xylan-Derived Carbohydrates
Abstract
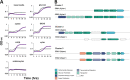
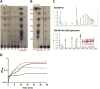
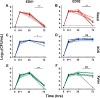
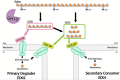
Xylans, a family of xylose-based polysaccharides, are dietary fibers resistant to digestion. They therefore reach the large intestine intact; there, they are utilized by members of the gut microbiota. They are initially broken down by primary degraders that utilize extracellular xylanases to cleave xylan into smaller oligomers. The resulting xylooligosaccharides (XOS) can either be further metabolized directly by primary degraders or cross-feed secondary consumers, including Bifidobacterium. While several Bifidobacterium species have metabolic systems for XOS, most grow poorly on longer-chain XOS and xylan substrates. In this study, we isolated strains of Bifidobacterium pseudocatenulatum and observed that some, including B. pseudocatenulatum ED02, displayed growth on XOS with a high degree of polymerization (DP) and straight-chain xylan, suggesting a primary degrader phenotype that is rare in Bifidobacterium. In silico analyses revealed that only the genomes of these xylan-fermenting (xylan+) strains contained an extracellular GH10 endo-β-1.4 xylanase, a key enzyme for primary degradation of xylan. The presence of an extracellular xylanase was confirmed by the appearance of xylan hydrolysis products in cell-free supernatants. Extracellular xylanolytic activity was only detected in xylan+ strains, as indicated by the production of XOS fragments with a DP of 2 to 6, identified by thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC). Additionally, in vitro fecal fermentations revealed that strains with a xylan+ phenotype can persist with xylan supplementation. These results indicate that xylan+ B. pseudocatenulatum strains may have a competitive advantage in the complex environment of the gastrointestinal tract, due to their ability to act as primary degraders of xylan through extracellular enzymatic degradation. IMPORTANCE The beneficial health effects of dietary fiber are now well established. Moreover, low fiber consumption is associated with increased risks of metabolic and systemic diseases. This so-called "fiber gap" also has a profound impact on the composition of the gut microbiome, leading to a disrupted or dysbiotic microbiota. Therefore, understanding the mechanisms by which keystone bacterial species in the gut utilize xylans and other dietary fibers may provide a basis for developing strategies to restore gut microbiome function. The results described here provide biochemical and genetic evidence for primary xylan utilization by human-derived Bifidobacterium pseudocatenulatum and show also that cooperative utilization of xylans occurs among other members of this species.
Keywords: bifidobacteria; cooperation; glycoside hydrolase; prebiotic; xylan; xylanase; xylooligosaccharide.
Conflict of interest statement
The authors declare a conflict of interest. R.H. has received grants and honoraria from several food and ingredient companies, is a co-owner of Synbiotic Health, and was on the Board of Directors of the International Scientific Association for Probiotics and Prebiotics.
Figures

References
-
- Bajpai P. 2014. Xylanolytic enzymes. Elsevier Inc, Amsterdam, The Netherlands.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

