Spontaneous Prophage Induction Contributes to the Production of Membrane Vesicles by the Gram-Positive Bacterium Lacticaseibacillus casei BL23
- PMID: 36200778
- PMCID: PMC9600169
- DOI: 10.1128/mbio.02375-22
Spontaneous Prophage Induction Contributes to the Production of Membrane Vesicles by the Gram-Positive Bacterium Lacticaseibacillus casei BL23
Abstract
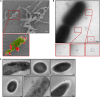
The formation of membrane vesicles (MVs) by Gram-positive bacteria has gained increasing attention over the last decade. Recently, models of vesicle formation have been proposed and involve the digestion of the cell wall by prophage-encoded or stress-induced peptidoglycan (PG) hydrolases and the inhibition of PG synthesis by β-lactam antibiotics. The impact of these mechanisms on vesicle formation is largely dependent on the strain and growth conditions. To date, no information on the production of vesicles by the lactobacilli family has been reported. Here, we aimed to characterize the MVs released by the Gram-positive bacteria Lacticaseibacillus casei BL23 and also investigated the mechanisms involved in vesicle formation. Using electron microscopy, we established that the size of the majority of L. casei BL23 vesicles ranged from 50 to 100 nm. Furthermore, we showed that the vesicles were released consistently throughout the growth of the bacteria in standard culture conditions. The protein composition of the vesicles released in the supernatant was identified and a significant number of prophage proteins was detected. Moreover, using a mutant strain harboring a defective PLE2 prophage, we were able to show that the spontaneous and mitomycin-triggered induction of the prophage PLE2 contribute to the production of MVs by L. casei BL23. Finally, we also demonstrated the influence of prophages on the membrane integrity of bacteria. Overall, our results suggest a key role of the prophage PLE2 in the production of MVs by L. casei BL23 in the absence or presence of genotoxic stress. IMPORTANCE The last few decades have demonstrated that membrane vesicles (MVs) produced by microorganisms can have a wide variety of functions. This diversity places MVs at the crossroads of major research topics in current microbiology such as antibiotic resistance, horizontal gene transfer, cell communication, biofilm development, bacteriophage resistance, and pathogenesis. In particular, vesicles produced by probiotic strains have been shown to play a significant role in their beneficial effects. Thus, the study of vesicle biogenesis is a key element for promoting and improving their release. Overall, our results suggest a key role of spontaneous and mitomycin-triggered prophage induction in MV production by the Gram-positive bacteria Lacticaseibacillus casei BL23. This phenomenon is of great interest as prophage-induced MVs could potentially influence bacterial behavior, stress resistance, and vesicle functions.
Keywords: Lacticaseibacillus casei; membrane vesicle production; membrane vesicles; prophages; spontaneous prophage induction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
