Early defoliation induces auxin redistribution, promoting paradormancy release in pear buds
- PMID: 36200868
- PMCID: PMC9706473
- DOI: 10.1093/plphys/kiac426
Early defoliation induces auxin redistribution, promoting paradormancy release in pear buds
Erratum in
-
Correction to: Early defoliation induces auxin redistribution, promoting paradormancy release in pear buds.Plant Physiol. 2023 Mar 17;191(3):2066. doi: 10.1093/plphys/kiad039. Plant Physiol. 2023. PMID: 36718628 Free PMC article. No abstract available.
Abstract
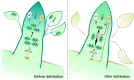
Paradormancy of fruit trees occurs in summer and autumn when signals from adjacent organs stimulate buds to develop slowly. This stage has received less attention that the other stages of dormancy, and the underlying mechanism remains uncharacterized. Early defoliation in late summer and early autumn is usually followed by out-of-season blooming in pear (Pyrus spp.), which substantially decreases the number of buds the following spring and negatively affects fruit production. This early bud flush is an example of paradormancy release. Here, we determined that flower bud auxin content is stable after defoliation; however, polar distribution of the pear (Pyrus pyrifolia) PIN-FORMED auxin efflux carrier 1b (PpyPIN1b) implied that auxin tends to be exported from buds. Transcriptome analysis of floral buds after artificial defoliation revealed changes in auxin metabolism, transport, and signal transduction pathways. Exogenous application of a high concentration of the auxin analog 1-naphthaleneacetic acid (300 mg/L) suppressed PpyPIN1b expression and its protein accumulation in the cell membrane, likely leading to decreased auxin efflux from buds, which hindered flower bud sprouting. Furthermore, carbohydrates and additional hormones also influenced out-of-season flowering. Our results indicate that defoliation-induced auxin efflux from buds accelerates bud paradormancy release. This differs from release of apical-dominance-related lateral bud paradormancy after the apex is removed. Our findings and proposed model further elucidate the mechanism underlying paradormancy and will help researchers to develop methods for inhibiting early defoliation-induced out-of-season bud sprouting.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures






References
-
- Andre D, Marcon A, Lee KC, Goretti D, Zhang B, Delhomme N, Schmid M, Nilsson O (2022) FLOWERING LOCUS T paralogs control the annual growth cycle in Populus trees. Curr Biol 32: 1–9 - PubMed
-
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51: 1019–1029 - PubMed
-
- Azeez A, Miskolczi P, Tylewicz S, Bhalerao RP (2014) A tree ortholog of APETALA1 mediates photoperiodic control of seasonal growth. Curr Biol 24: 717–724 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

