Superinfection with SARS-CoV-2 Has Deleterious Effects on Mycobacterium bovis BCG Immunity and Promotes Dissemination of Mycobacterium tuberculosis
- PMID: 36200898
- PMCID: PMC9603897
- DOI: 10.1128/spectrum.03075-22
Superinfection with SARS-CoV-2 Has Deleterious Effects on Mycobacterium bovis BCG Immunity and Promotes Dissemination of Mycobacterium tuberculosis
Abstract
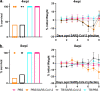
An estimated one-third of the world's population is infected with Mycobacterium tuberculosis, with the majority being vaccinated with Mycobacterium bovis BCG. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains a threat, and we must understand how SARS-CoV-2 can modulate both BCG immunity and tuberculosis pathogenesis. Interestingly, neither BCG vaccination nor tuberculosis infection resulted in differences in clinical outcomes associated with SARS-CoV-2 in transgenic mice. Surprisingly, earlier M. tuberculosis infection resulted in lower SARS-CoV-2 viral loads, mediated by the heightened immune microenvironment of the murine lungs, unlike vaccination with BCG, which had no impact. In contrast, M. tuberculosis-infected tissues had increased bacterial loads and decreased histiocytic inflammation in the lungs following SARS-CoV-2 superinfection. SARS-CoV-2 modulated BCG-induced type 17 responses while decreasing type 1 and increasing type 2 cytokines in M. tuberculosis-infected mice. These findings challenge initial findings of BCG's positive impact on SARS-CoV-2 infection and suggest potential ramifications for M. tuberculosis reactivation upon SARS-CoV-2 superinfection. IMPORTANCE Prior to SARS-CoV-2, M. tuberculosis was the leading infectious disease killer, with an estimated one-third of the world's population infected and 1.7 million deaths a year. Here, we show that SARS-CoV-2 superinfection caused increased bacterial dissemination in M. tuberculosis-infected mice along with immune and pathological changes. SARS-CoV-2 also impacted the immunity of BCG-vaccinated mice, resulting in decreased interleukin-17 (IL-17) levels, while offering no protective effect against SARS-CoV-2. These results demonstrate that SARS-CoV-2 may have a deleterious effect on the ongoing M. tuberculosis pandemic and potentially limit BCG's efficacy.
Keywords: BCG; Mycobacterium tuberculosis; SARS-CoV-2; pathogenesis; superinfection; virulence.
Conflict of interest statement
The authors declare a conflict of interest. A.M.T. has financial interests in Pan Genome Systems, Inc., a startup company developing animal and human vaccines.
Figures








Similar articles
-
Listeria-Vectored Multiantigenic Tuberculosis Vaccine Enhances Protective Immunity against Aerosol Challenge with Virulent Mycobacterium tuberculosis in BCG-Immunized C57BL/6 and BALB/c Mice.mBio. 2022 Jun 28;13(3):e0068722. doi: 10.1128/mbio.00687-22. Epub 2022 Jun 1. mBio. 2022. PMID: 35642945 Free PMC article.
-
Mycobacterium bovis BCG immunization induces protective immunity against nine different Mycobacterium tuberculosis strains in mice.Infect Immun. 2008 Nov;76(11):5173-80. doi: 10.1128/IAI.00019-08. Epub 2008 Aug 18. Infect Immun. 2008. PMID: 18710860 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Increased pulmonary tumor necrosis factor alpha, interleukin-6 (IL-6), and IL-17A responses compensate for decreased gamma interferon production in anti-IL-12 autovaccine-treated, Mycobacterium bovis BCG-vaccinated mice.Clin Vaccine Immunol. 2011 Jan;18(1):95-104. doi: 10.1128/CVI.00352-10. Epub 2010 Nov 17. Clin Vaccine Immunol. 2011. PMID: 21084465 Free PMC article.
-
Pathogenesis of SARS-CoV-2 and Mycobacterium tuberculosis Coinfection.Front Immunol. 2022 Jun 16;13:909011. doi: 10.3389/fimmu.2022.909011. eCollection 2022. Front Immunol. 2022. PMID: 35784278 Free PMC article. Review.
Cited by
-
Tackling Drug-Resistant Tuberculosis: New Challenges from the Old Pathogen Mycobacterium tuberculosis.Microorganisms. 2023 Sep 10;11(9):2277. doi: 10.3390/microorganisms11092277. Microorganisms. 2023. PMID: 37764122 Free PMC article. Review.
-
Neuropathological lesions in intravenous BCG-stimulated K18-hACE2 mice challenged with SARS-CoV-2.Vet Res. 2024 May 31;55(1):71. doi: 10.1186/s13567-024-01325-7. Vet Res. 2024. PMID: 38822398 Free PMC article.
-
Bacterial-induced or passively administered interferon gamma conditions the lung for early control of SARS-CoV-2.Nat Commun. 2023 Dec 12;14(1):8229. doi: 10.1038/s41467-023-43447-0. Nat Commun. 2023. PMID: 38086794 Free PMC article.
-
Animal models for COVID-19 and tuberculosis.Front Immunol. 2023 Aug 11;14:1223260. doi: 10.3389/fimmu.2023.1223260. eCollection 2023. Front Immunol. 2023. PMID: 37638020 Free PMC article. Review.
-
Mycobacterium tuberculosis and SARS-CoV-2 co-infections: The knowns and unknowns.iScience. 2023 May 19;26(5):106629. doi: 10.1016/j.isci.2023.106629. Epub 2023 Apr 8. iScience. 2023. PMID: 37091987 Free PMC article. Review.
References
-
- World Health Organization. 2011. Global tuberculosis control. WHO Press, Geneva, Switzerland.
-
- World Health Organization. 2021. WHO coronavirus (COVID-19) dashboard. WHO health emergency dashboard. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

